Key Trends in Aging Research: Where Are We Now?
Over the past decade, aging research has transitioned from foundational biological studies, including a landmark introduction of 9 hallmarks of aging back in 2013 and its expanded version of 12 hallmarks in 2023, to a highly technical, multidisciplinary field. This transformation has been driven by a lot of advances in biology, but also technological innovations, particularly in artificial intelligence (AI), which enabled more sophisticated biomarker discovery, and clinical interventions.
Below, I would like to highlight some major trends shaping this field, based on the latest insights from a comprehensive article “Longevity biotechnology: bridging AI, biomarkers, geroscience and clinical applications for healthy longevity” which was published as a collective work initiated during 2023 Aging Research and Drug Discovery (ARDD) meeting. I would also share some thoughts about what can probably be done today for “an average person”.
I was introduced to the field of aging research quite recently, when I first visited a high profile conference, 11th Aging Research and Drug Discovery Meeting (ARDD2024) in the city of Copenhagen at the end of August. It was a very pleasant experience, both organization-wise and content-wise, but this post is not about that.
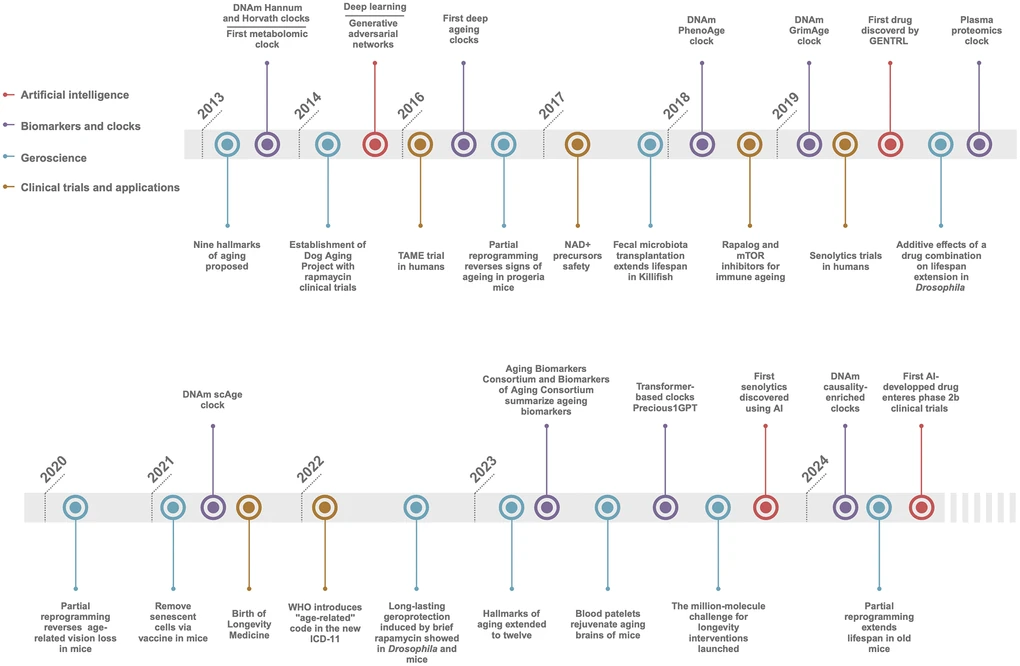
image source: https://www.aging-us.com/article/206135/text
I found that the aging research industry has a lot of potential with a diverse set of interventions in preclinical stages, successes in animal models, and even some ongoing clinical trials which are kind of related to aging. However, I was left with an impression that as of now, there seems to be not a lot that is available to the general public beyond traditional “sleep well, eat well, exercise, run yearly checkups, and socialize” kinds of things (aka “what mother told you”). There are some exceptions, with a number of potentially beneficial remedies available for a few of those who possess sufficient domain knowledge to really understand and manage the risk-to-benefit ratio. Results are also not proven definitively, but are quite likely.
So, we are in very early days of real longevity for everyone, but it is also true that we may expect some major advances fairly soon and quite unexpectedly (or not, who really knows).
Anyway, let’s get to some insights from the article:
1. Artificial Intelligence in Aging Research (2013-2023)
The integration of machine learning algorithms, deep learning techniques, and large-scale data analytics into biomarker research has enabled the identification and validation of aging biomarkers that are critical for diagnosing diseases, forecasting outcomes, and tailoring treatments.
For instance, deep learning applied to cellular images from various tissues has revealed nuclear morphology as a new, universal marker of cellular senescence. However, relying on single biomarkers is often insufficient for accurately tracking aging processes. Instead, the use of biomarker panels or composite biomarkers that combine data from multiple ‘omics’ technologies—such as genomics, proteomics, and metabolomics—offers a more comprehensive approach.
Additionally, the use of explainable AI is crucial in developing models that predict chronological age using non-invasive techniques. In the future, AI's integration with advanced technologies like single-cell sequencing and spatial transcriptomics will likely drive forward biomarker discovery, particularly for complex, multifactorial diseases. Importantly, the focus is shifting toward identifying therapeutic biomarkers that not only predict the onset of age-related diseases but also track responses to gerotherapeutic interventions—treatments designed to delay, prevent, or alleviate aging-related conditions while extending healthspan.
Companies such as Insilico Medicine, BioAge Labs and Deep Longevity are integrating AI for drug discovery and diagnostics with the aging processes in mind, particularly focusing on understanding the genetic and epigenetic mechanisms underlying longevity. Their work highlights AI’s potential in speeding up the drug discovery process by rapidly testing compounds in silico before transitioning to laboratory trials.
For instance, Insilico Medicine managed to build a pipeline of clinical candidates with an average concept-to-preclinical candidate nomination timescales being around 18 months. It is not a typical timeline for a classical research model and a case that I discussed in the pipeline analysis of the 10 AI in drug discovery platform around a year ago.
It should be noted, that most drug candidates in the Insilico Medicine’s pipeline are not actually longevity interventions, rather, interventions for broad age-related diseases, like fibrosis, with novel targets that are potentially relevant to aging research. The problem here is that aging is not a disease in the modern classification, and so it is hard to build a clinical stage company around targeting aging, even if the research may have high relevance to aging processes.
When I asked a question during a panel discussion at ARDD2024 about how companies in the aging research field should go about building pipelines, the answer was quite unexpected. I did not record the answer, but If I remember it correctly, Alex Zhavoronkov said that it is in fact pretty hard to do, so novel biotech companies with ambitions in the aging field should go after “legitimate diseases” to make it happen as a business, structure trials and so on.
But they should try to discover novel and broad targets with high relevance to aging processes. This way, on the one hand, they will have clear path via standard clinical regulatory pathways like everyone else (hence, investments, and understandable business and milestones), but will also be accumulating new knowledge and know-how about aging processes to have an edge when the industry has clear pathways to go about aging research as a disease.
2. Advancements in Biomarker Discovery and Aging Clocks
The discovery of biomarkers - measurable indicators of biological processes - has been instrumental in aging research. These biomarkers allow for the tracking of biological aging, which is distinct from chronological aging. Since the development of the first DNA methylation clocks by Steve Horvath in 2013, there has been significant progress in creating more sophisticated aging clocks that integrate various omics data (e.g., genomics, proteomics, metabolomics).
For example, the OMICmAge clock, introduced in 2023, integrates data from proteomics, metabolomics, clinical information, and DNA methylation to predict disease risk and lifespan. This clock has demonstrated improved accuracy in predicting 5-year and 10-year survival rates compared to earlier generations of aging clocks.
Additionally, platforms like Biolearn, Clockbase, and methylCIPHER are dedicated to the development and validation of aging biomarkers, providing researchers with standardized datasets and benchmarks for comparing and improving existing biomarkers. This standardization is crucial for transitioning biomarkers from research labs to clinical practice.
3. The Twelve Hallmarks of Aging: Mechanisms and Therapeutic Targets
As I mentioned in the introduction, aging research has evolved to identify the twelve hallmarks of aging, which include molecular damage (e.g., genome instability, telomere attrition), and cellular processes (e.g., autophagy, proteostasis). These hallmarks, identified through extensive research, provide a comprehensive framework for understanding how aging manifests at the molecular, cellular, and systemic levels.
Research on genome instability, for instance, has highlighted that accumulated DNA damage and the shortening of telomeres are key drivers of aging. Notable research from Ercc1 mutant mice — a model of progeroid syndrome — has demonstrated that particular DNA repair gene mutations can accelerate aging processes, initiating other hallmarks of aging, such as cellular senescence and inflammation.
Epigenetic alterations have emerged as another critical hallmark. Recent advancements in partial reprogramming — the temporary use of Yamanaka factors to reset cells to a more youthful state—have shown potential in reversing age-related damage. This method, however, comes with risks, such as disrupting cell identity or increasing the risk of cancer, thus requiring careful application in therapeutic settings.
In the area of autophagy — the cell’s recycling process — AI has been used to identify potential drug candidates that target mitophagy (the selective degradation of mitochondria), which has shown promising results in preclinical models of Alzheimer’s disease.
4. Senolytics and Cellular Senescence
Cellular senescence—when cells cease dividing and release pro-inflammatory factors—plays a pivotal role in aging. Removing these senescent cells has become a primary target in anti-aging therapies. The development of senolytics, a class of drugs designed to selectively eliminate senescent cells, has shown promise in preclinical and early clinical studies.
Dasatinib (a cancer drug) and quercetin (a natural compound) were among the first senolytics to demonstrate efficacy in reducing senescence markers and improving health in animal models. Unity Biotechnology, founded by Nathanial “Ned” David, is active in senolytic drug development for eye diseases, and has led clinical trials targeting diseases of aging by eliminating senescent cells.
Other innovative approaches include senomorphics, which modulate the behavior of senescent cells without killing them, and the use of extracellular vesicles (EVs) and CAR-T therapies to remove or rejuvenate senescent cells. These technologies are actively being investigated to determine their safety and efficacy in humans, with an emphasis on their potential in treating chronic age-related diseases.
5. Mitochondrial Dysfunction and Nutrient Sensing Pathways
Research into mitochondrial dysfunction and nutrient sensing pathways has revealed these systems’ critical roles in regulating aging. Mitochondrial dysfunction leads to decreased energy production and increased oxidative stress, contributing to cellular damage. Therapeutic interventions targeting mitochondrial health, such as NAD+ precursors and natural compounds like trigonelline, have been explored for their potential to restore mitochondrial function.
The mTOR and AMPK pathways, which control how cells respond to nutrient levels, are also central to aging. Inhibition of mTOR by drugs like rapamycin has shown promise in extending lifespan by reducing protein synthesis and promoting autophagy. Additionally, metformin, a diabetes drug, has been repurposed to target aging through its effects on nutrient sensing and metabolism. The ongoing TAME (Targeting Aging with Metformin) trial, led by Dr. Nir Barzilai, seeks to test whether metformin can extend lifespan and delay age-related diseases in humans.
6. Clinical Translation and Gerotherapeutics
Despite significant laboratory advances, clinical translation remains one of the most challenging aspects of aging research. The process of moving from discovery to clinical application requires rigorous validation and harmonization of biomarkers, as well as overcoming regulatory hurdles. Initiatives like Biolearn, Estimage, and Clockbase aim to facilitate this process by providing standardized metrics and validation platforms for aging biomarkers.
In clinical settings, there is growing interest in gerotherapeutics — therapies designed to target the mechanisms of aging. Many clinical trials currently focus on interventions that target antagonistic hallmarks, such as deregulated nutrient sensing and mitochondrial dysfunction, as these processes are said to have shown the most promise in preclinical studies.
7. Longevity Medicine and Personalized Healthcare
The emergence of longevity medicine represents a shift from traditional disease-focused healthcare to a preventive model that integrates deep biomarkers of aging. Personalized medicine platforms, such as InsideTracker and Singulomics, use AI to combine blood biomarkers, DNA data, and physiological metrics to create individualized longevity strategies.
Great… So What?
The field of aging research is booming, there is no doubt. I would not dare predict where the first instance of sustainable “radical longevity” will come from, but I think it may very well happen within years from now, not decades. It is an interesting space to watch and participate, whether you are a researcher, an investor or a science blogger like me.
Now, speaking about practical conclusions for an “average person.” What do we do now, while “all the powerful stuff is still being tested in mice”?
There are quite actionable drugs known to show anti-aging effects, available today. A good example is rapamycin. But as I said earlier, I don’t think it is for an “average person” like myself. And even though technically speaking my expertise does allow me to comprehend the benefits and risks of this and other molecules if I really dive in, I am not ready to really go down that rabbit hole for now, especially with a medical system I have in the country of current residence, where it might be challenging tracking biomarkers all the time.
Rapamycin is not generally suitable for average use due to its potential toxicity and immunosuppressive effects. I can’t recommend something that I would personally not take, at least at this age, being relatively healthy. Maybe when I am 60 or 70, I would be more proactive and daring to experiment with my molecular pathways, but let’s hope we will have a clear and simple set of best practices by then!
A more practical approach for the average person today would probably be to focus on safe lifestyle interventions like exercise, dietary modifications (e.g., calorie restriction or intermittent fasting), sleep, regular medical checkups, and may be some natural supplements such as spermidine, NAD+ precursors, or urolithin-A, which have fewer safety concerns and are more easily integrated into daily routines.
But again, even supplements are not as harmless as one may think, so you have to consult your doctor… or I don’t know, Bryan Johnson maybe? Anyway, it is not medical advice. I am not a medical doctor, I am a chemistry PhD.
Topics: Aging & Longevity