Iambic Therapeutics Unveils Enchant AI to Predict Clinical Outcomes Using Preclinical Data
Iambic Therapeutics has announced the launch of Enchant, an AI platform designed to predict clinical outcomes from the earliest stages of drug discovery. Enchant is a multi-modal transformer model that aims to address a critical bottleneck in drug development by utilizing preclinical data to improve predictions about clinical outcomes, even when clinical data is limited. The model is trained across numerous data modalities and sources, spanning the full drug discovery and development process.
The main challenge Enchant is intended to address is the "data wall" between preclinical and clinical stages. Preclinical discovery generates large volumes of data on thousands of molecules, but only a small number progress to clinical trials, where data becomes scarce. Enchant is designed to leverage discovery-stage data to predict clinical properties, such as pharmacokinetics (PK), which describe how the body processes a drug.
Tom Miller, PhD, Iambic’s Chief Executive Officer, explained:
“Bringing a drug to market often costs billions, partly because critical pharmacological insights aren’t uncovered until human trials are well underway. Enchant is designed to cut years from preclinical development, speed up trial timelines, and prevent late-stage discoveries of liabilities that can jeopardize clinical success.”
The white paper presents Enchant’s predictive capabilities, showing that it can provide reliable predictions of human PK properties even when trained on less than 1% of the Obach human PK dataset. As further presented, competitor technologies fail to yield accurate predictions with such limited training data. With additional preclinical data, Enchant’s performance improves significantly, achieving a Spearman correlation coefficient of 0.74 for human PK half-life, compared to the previous state-of-the-art of 0.58.
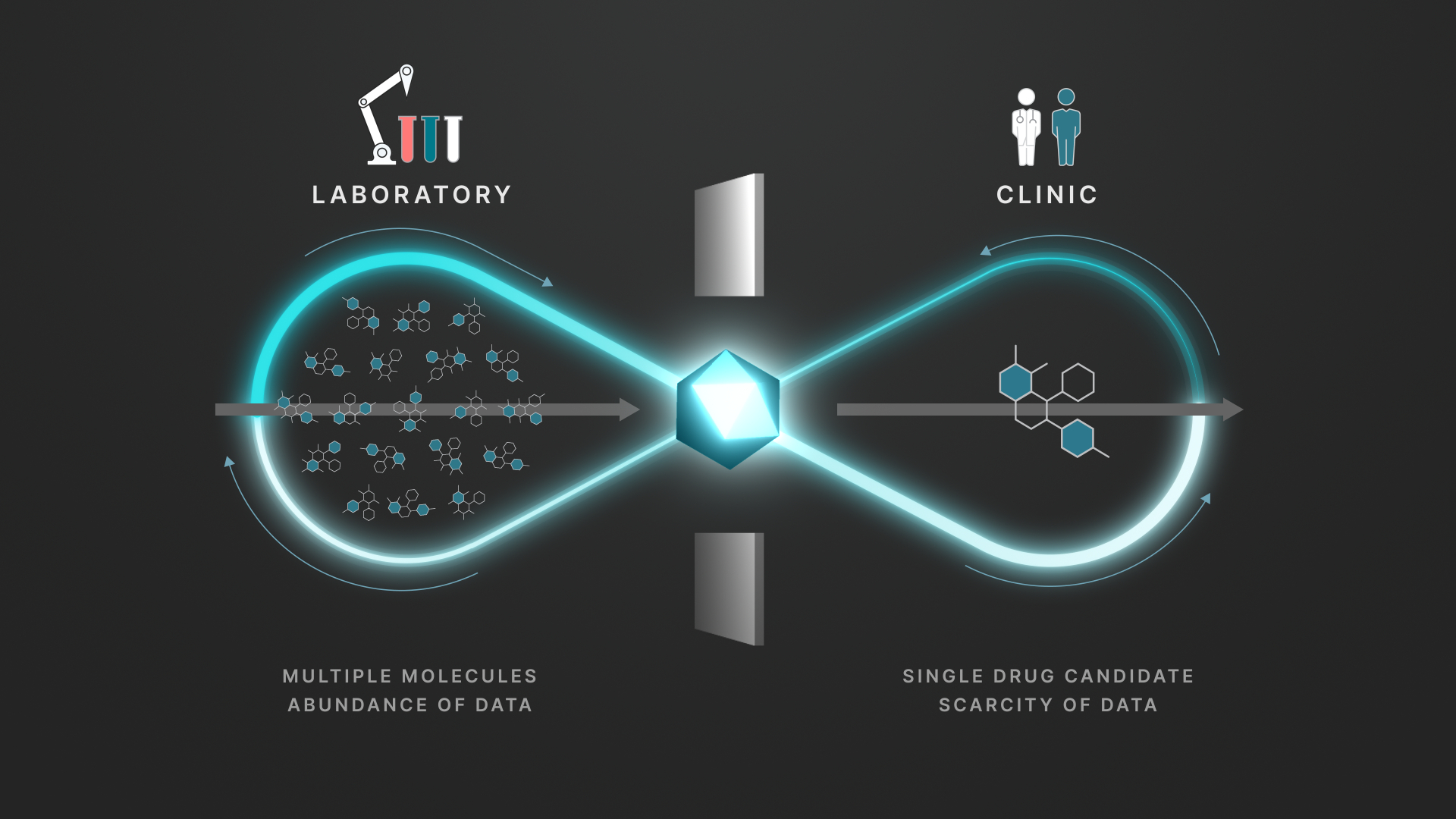
The Enchant diagram shows how it uses discovery-stage data to predict clinical outcomes when clinical data is limited.
Fred Manby, PhD, Iambic’s Chief Technology Officer, emphasized:
“Enchant addresses a fundamental data bottleneck in drug discovery, supplementing scarce clinical data with readily generated and abundant preclinical laboratory data. When trained on the full set of available human clinical PK data, Enchant’s clinical predictions surpass all state-of-the-art models. But most critically the model gets better at predicting clinical outcomes by training on more laboratory data.”
By incorporating preclinical data and even small amounts of clinical data, Enchant aims to help researchers make more informed decisions, potentially reducing the time and cost associated with drug development while improving the probability of clinical success.
Topics: Clinical Trials