UK-based Laverock Therapeutics Expands Gene-Silencing Technology to Tackle Multiple Disease Areas
Laverock Therapeutics, a UK company based at the Stevenage Bioscience Catalyst, is advancing its programmable gene-silencing platform, GEiGS, aimed at simultaneously silencing multiple genes. The technology uses reprogrammed microRNAs (miRNAs)—molecules that naturally regulate gene expression—to silence genes in a precise, tunable, and stable manner, expanding its potential applications in oncology and genetic diseases like Charcot-Marie-Tooth disease, a hereditary nerve disorder.
Key points:
-
Company Focus: Development of programmable gene-silencing therapies, primarily targeting oncology and genetic diseases. The platform re-directs microRNAs to silence genes in a specific manner.
-
Technology Progress: The company demonstrated gene silencing across multiple genes and cell types, including simultaneous silencing of multiple genes. Key targets include B2M (MHC-I complex) and seven additional validated targets.
-
Industrialisation: Laverock increased platform throughput for screening, gene editing, and cell line development, supporting in-house product development and partnering opportunities.
-
Pipeline Focus: Laverock’s therapeutic pipeline includes oncology and genetic diseases. They aim to expand into areas like type 1 diabetes, hypoimmunogenicity, fibrosis, inflammation, and regenerative medicine through partnerships.
-
Future Plans: The company is actively seeking partners for broader applications of its technology and has initiated a financing round to fund ongoing pipeline and platform advancements.
Laverock demonstrated its ability to silence two target genes in human induced pluripotent stem cells (iPS cells)—GFP (a fluorescent marker) and B2M (a key component of the immune system)—showing high correlation in silencing efficiency between mono- and bi-allelic gene edits.
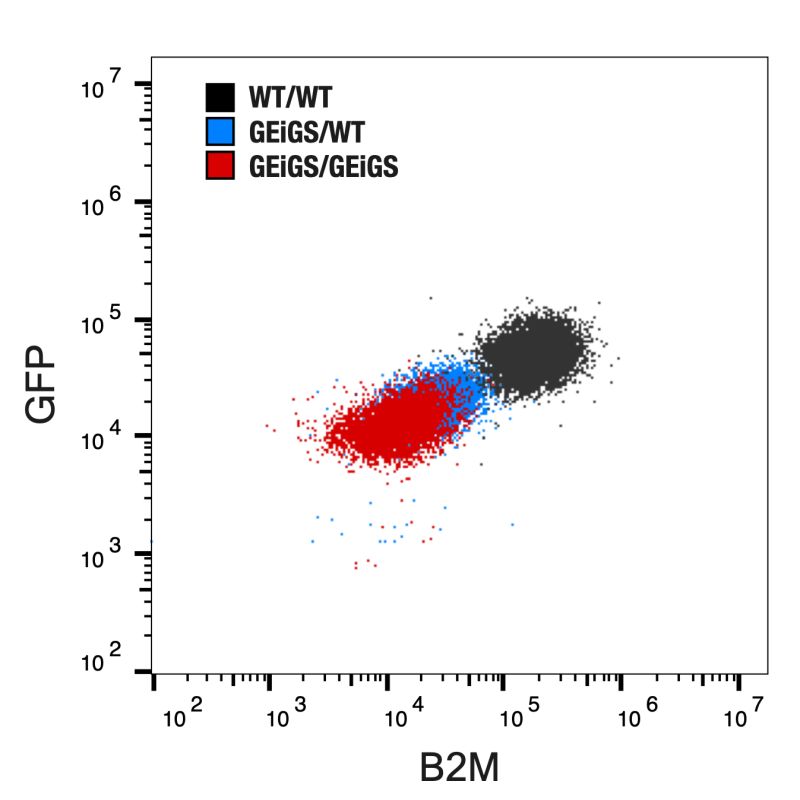
Simultaneous silencing of two different targets using Laverock’s gene silencing RNAs. Source: Laverock Therapeutics
Laverock has also scaled up its gene-silencing platform by improving computational tools and automating gene editing workflows. The company reports that screening capacity has increased by two orders of magnitude since 2023. Previously able to screen only 20 gene-silencing RNA designs per target, they now screen 6,000 RNA designs across eight targets. This leap is expected to accelerate the development of new therapeutic candidates, as it allows for a wider array of targets to be evaluated in a shorter time frame.
Automation of gene editing and cell line development has also been optimized through the implementation of liquid handling-based systems, leading to a 10x increase in capacity over manual workflows. This automation allows Laverock to quckly identify preferred cell lines in downstream functional assays, speeding up the process from screening to clinical application.
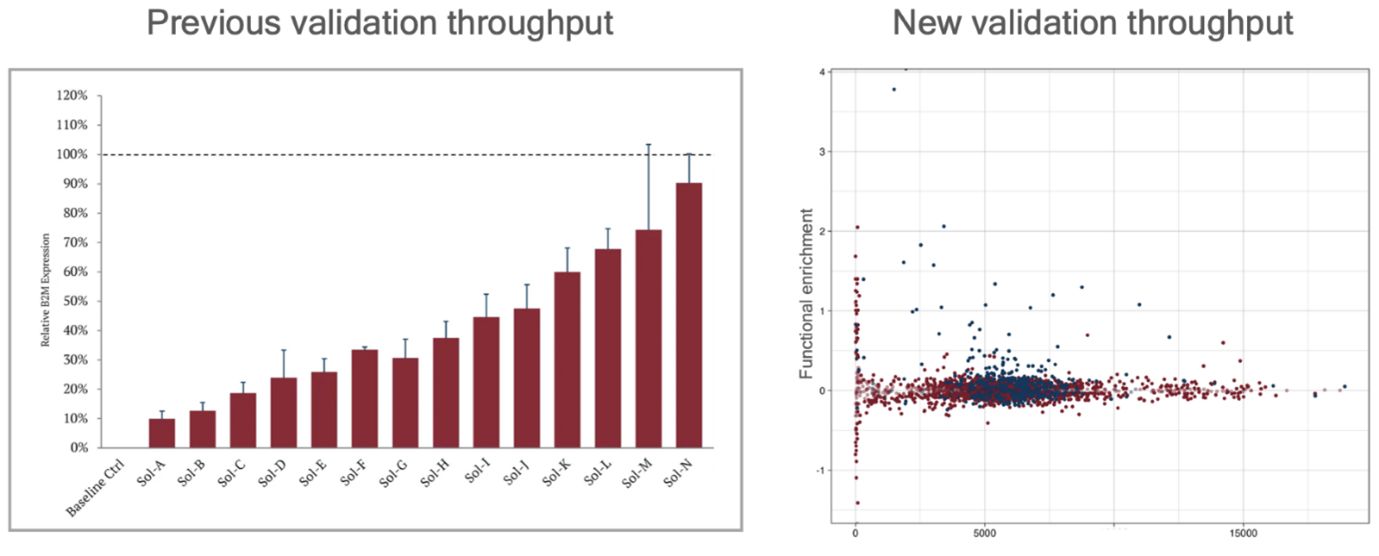
Previous vs. new validation throughput. Source: Laverock Therapeutics
Laverock's internal pipeline focuses on engineered T-cells and iPSC-derived macrophages for treating solid tumors. The goal is to create cell therapies that resist the immunosuppressive tumor microenvironment, which usually limits the effectiveness of such treatments. Additionally, Laverock is developing hypoimmunogenic pancreatic islet cells to treat Type 1 diabetes, designed to prevent immune rejection and improve therapeutic outcomes.
In terms of therapeutic flexibility, the company claims its gene-silencing platform can target both endogenous (naturally occurring) and exogenous (foreign) genes, opening up possibilities for treating conditions that are not effectively addressed by current therapies. This platform is compatible with patient-derived and donor-derived product development, with a key advantage being the minimal gene edits required, avoiding the need for incorporating non-human sequences.
The company is seeking partners to explore broader applications, including treatments for fibrosis, inflammation, and regenerative medicine, while continuing to validate its technology for both cell-based and in vivo therapies.
Cover photo: Laverock Therapeutics
Topics: Biotech