Three Big Ideas in Aging Research That Could Shift the Therapeutic Landscape
Disclaimer: this post is not medical advice; the content of the post is for informational and educational purposes only.
Over the past decade, aging research has transitioned from a mostly fundamental science practice, including a landmark introduction of 9 hallmarks of aging back in 2013 and its expanded version of 12 hallmarks in 2023, to a highly technical, multidisciplinary field with increasingly tangible practical potential. This transformation is happening thanks to numerous advances in the biology of aging, including the emergence of age-specific biomarkers or “aging clocks”, senolitics, cell reprogramming, and many other promising directions. But it is also enabled by a rapid advent of foundational technologies, such as artificial intelligence (AI), which opened doors for more sophisticated biology modeling, better understanding aging processes, and potentially uncovering new creative ideas towards solving the human life’s current biggest limitation: an inevitable decline and end.
Below, I would like to highlight several recent advances in aging research that can redefine the landscape of therapeutics development in this area, both R&D and business-wise. This is based on learnings from this year’s 12th Aging Research and Drug Discovery Meeting (ARDD2025) in Copenhagen, and my coverage of last year's ARDD2024 event.
Is the First Anti-aging Drug Class on the Horizon?
During this year’s Aging Research and Drug Discovery Meeting, Andrew Adams, Group Vice President of Molecule Discovery at Eli Lilly, presented a thought-provoking case GLP-1 receptor agonists as potentially the first class of longevity therapeutics, with both mechanistic relevance and clinical scale to be able to impact human healthspan on a global population scale.
Traditionally indicated for type 2 diabetes and obesity, GLP-1s such as semaglutide and tirzepatide have shown robust, multi-system benefits: reducing the progression from pre-diabetes to diabetes by up to 94%, significantly lowering cardiovascular events (MACE), and producing weight loss with downstream metabolic and inflammatory effects.

Image taken during ARDD2025.
Adams emphasized the relevance of these outcomes in delaying the onset of chronic, age-related diseases, thus compressing morbidity, a core objective in longevity medicine. He further highlighted early signals suggesting GLP-1s may exert positive effects on vascular function, cognitive decline, and even psychiatric and addiction-related conditions, though he acknowledged that more evidence is needed in these emerging indications.
Importantly, he framed these drugs within a broader shift from “sick care” to proactive, preventive healthcare, suggesting that long-acting delivery formats, such as RNA-based or gene-editing approaches, could further extend their reach and adherence in aging populations.
While he did not claim GLP-1s reverse aging or extend maximum lifespan, Adams posed a critical question: are GLP-1s the first true longevity drug class, considering their scalable, evidence-based impact across age-related disease trajectories?
A similar perspective was reinforced during the same conference by Dr. Lotte Bjerre Knudsen, Chief Scientific Advisor at Novo Nordisk and the scientific mind behind semaglutide. She opened her presentation with a striking and unambiguous title slide: “Semaglutide as a Proven Longevity Medicine,” as Alex Zhavoronkov, Co-founder and CEO of Insilico Medicine, writes in his LinkedIn post.
Now, zooming back from the burgeoning area of GLP-1s with all the promise of their therapeutic expansion into age-related diseases and even aging itself, let’s talk about a broader issue for the aging research field: how to cure something that is not a disease?
Finding the Missing Link Between Aging Research and Clinical Development
Aging may be the biggest risk factor for chronic disease, but it still isn’t recognized as a disease itself, which creates a major bottleneck for companies attempting to go after anti-aging therapies directly. Without a formal indication, there’s no clear regulatory path, no accepted clinical endpoints, and little incentive for venture capitalists to fund such “vague” endeavors. This situation, undoubtedly, inhibits the progress of the longevity field.
To get around this, co-authors of a recent paper published in Aging, Alex Zhavoronkov, Dominika Wilczok, Feng Ren, and Fedor Galkin, put forward a compelling idea: instead of trying to treat aging head-on as a business model, treat diseases that biologically mirror it. Their work suggests that some age-related diseases are far more representative of the aging process than others, and that these can serve as practical, high-fidelity proxies for testing geroprotective drugs.
To find the best candidates, they built a scoring system that ranks diseases based on how closely they align with the hallmarks of aging, processes like telomere shortening, mitochondrial dysfunction, cellular senescence, and deregulated nutrient sensing.
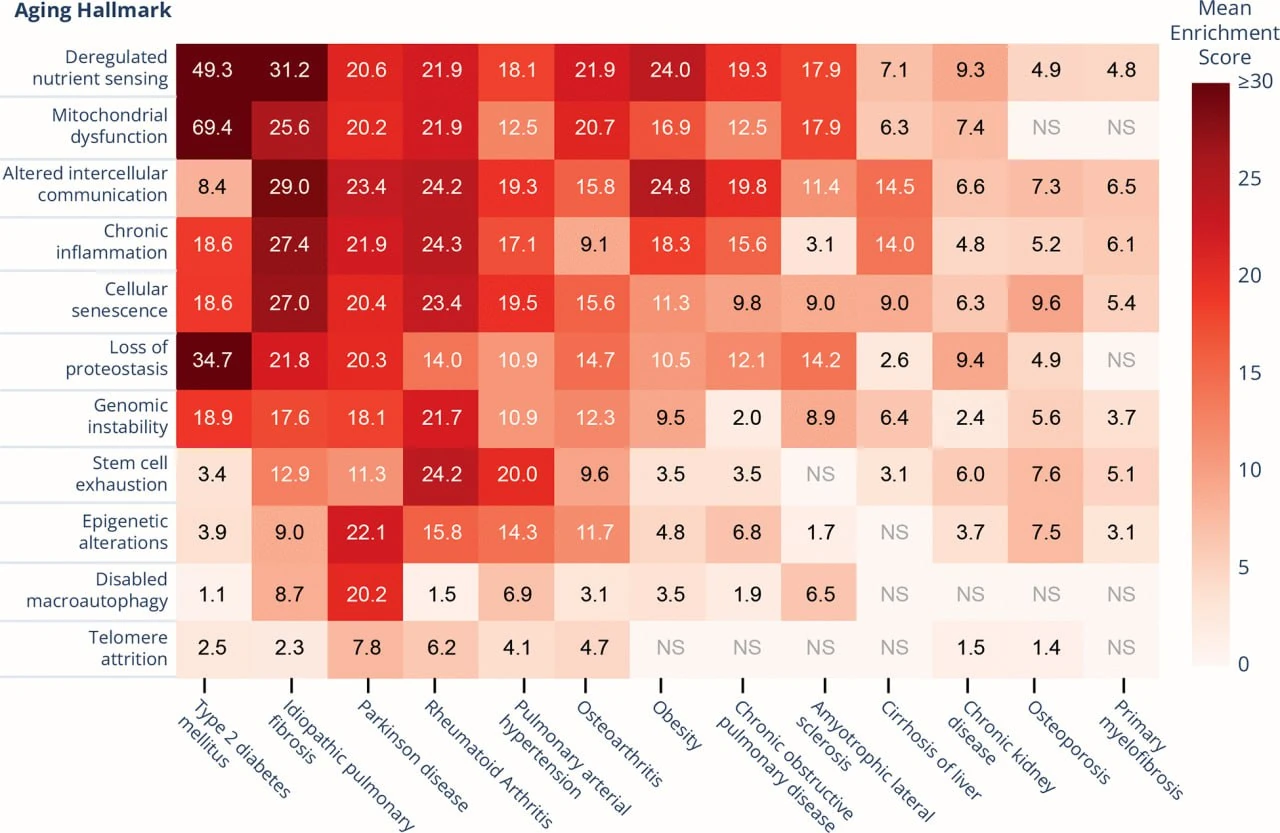
Out of 13 diseases evaluated, one stood out: idiopathic pulmonary fibrosis (IPF).
Not only does IPF score high across nearly every hallmark, but it also progresses quickly. It has well-defined clinical endpoints, making it uniquely suited for real-world trials of anti-aging therapies. In essence, IPF offers the aging field something it’s been missing: a tractable, measurable, and regulatorily viable way to bring geroprotectors into the clinic, even if indirectly.
This alignment is not just theoretical; it has already begun to influence drug development strategy. For example, Rentosertib, developed by Insilico Medicine and currently in Phase 2a clinical trial, inhibits TNIK and modulates Wnt/β-catenin signaling to reduce epithelial senescence and fibrosis.
The well-known senolytic combo dasatinib and quercetin is being tested for its ability to selectively clear senescent cells in IPF lungs. Danazol and cycloastragenol aim to restore telomerase activity, targeting telomere attrition, a hallmark strongly implicated in familial IPF.
And upstream, interventions like resveratrol and caloric restriction mimetics are being explored for their effects on sirtuins and epigenetic regulation.
For companies focused on longevity, IPF trials provide not only a clinically relevant path to market but also a powerful signal generator for anti-aging efficacy. A positive outcome in IPF can serve as both regulatory validation and scientific proof-of-concept, enabling indication expansion into other fibrotic or aging-aligned diseases such as chronic kidney disease, liver cirrhosis, or atherosclerosis. This approach may help work around the regulatory bottleneck of targeting "aging" directly and anchor aging drug development in diseases already recognized by regulators and payers.
Finally, the third big idea in aging research centers around a somewhat unintuitive topic in this context: gut microbiome.
Do Bacteria in Our Gut Hold the Key to Healthspan and Longevity?
At ARDD 2024, Scotch McClure, CEO of Maxwell Biosciences, a Texas-based AI-driven preclinical stage biotech, proposed a striking thesis: microbial imbalance (dysbiosis) may not just drive disease, it may actively accelerate biological aging. And reversing it, he argued, may offer a direct intervention point for extending healthspan.
The human body hosts over 39 trillion microbial cells, weighing approximately 1.5 to 2 kilograms, with the gut microbiome alone containing over 1,000 species of bacteria. Their collective genetic material outnumbers human genes by 150:1. When balanced, this ecosystem regulates immune response, metabolism, and tissue repair. But when disrupted, via pathogens, antibiotics, or poor diet, dysbiosis sets in, triggering chronic inflammation, immune dysregulation, and systemic aging.
McClure linked dysbiosis directly to all 12 hallmarks of aging, including genomic instability, telomere attrition, epigenetic alterations, mitochondrial dysfunction, cellular senescence, and loss of proteostasis. He cited evidence that dysbiosis impairs stem cell function, deregulates nutrient sensing, and accelerates immune decline (immunosenescence), leading to early onset of frailty, cognitive decline, and chronic disease.
In an exclusive interview for BiopharmaTrend, Scotch McClure said:
“The human microbiome expresses over 200x the human cell genetic expression. So the major lever in epigenetics is the microbiome. Therefore, aging should be at least partially treated as a communicable disease. Pathogens are a key driver of inflammaging, immunosenescence, stem cell depletion, heart disease, cancer, diabetes, and all-cause mortality. It is actually quite silly that we haven’t already categorized pathogens as a key driver of aging”.
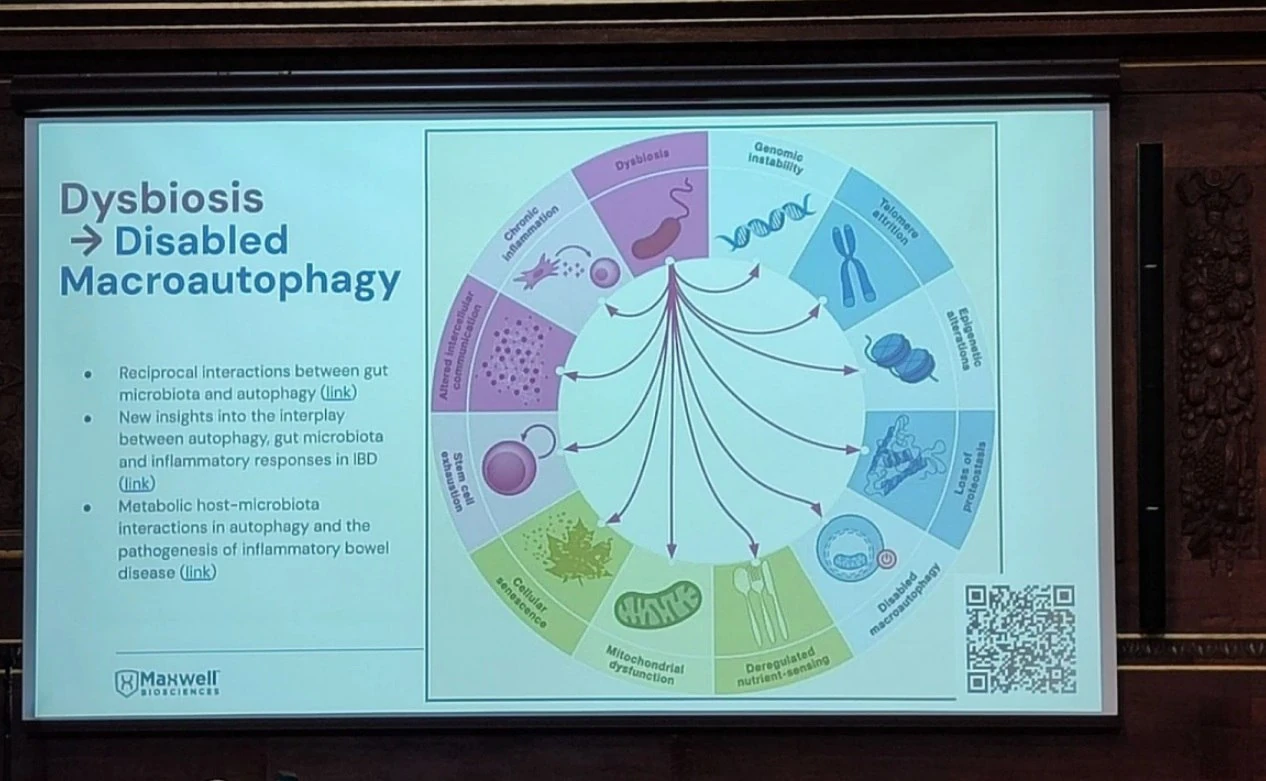
To counter this, Maxwell Biosciences is developing a synthetic analog of LL-37, a broad-spectrum antimicrobial peptide naturally produced by the immune system. While LL-37 is effective against bacteria, viruses, and fungi, its clinical potential is limited by instability and rapid degradation in vivo.
Using their proprietary CLAROMER® platform, Maxwell has created synthetic peptoids, poly-N-substituted glycines, that mimic LL-37’s structure and function, are stable at room temperature, require no cold chain logistics, show no resistance development in pathogen exposure studies, and do not disrupt the healthy gut microbiome.
These peptoids disrupt microbial membranes, including biofilms, while preserving host tissue. The company’s lead candidate will be delivered as a nasal spray, targeting the nasal-brain axis, an emerging interface between microbiota, inflammation, and neurodegeneration.
The broader ambition is to build a “synthetic immune system”: modular, tunable peptoid-based therapeutics that restore innate immunity, clear chronic pathogens, and reduce inflammation — addressing the microbial drivers of aging at their source.
The company plans its first clinical trials in 2026 and aims to expand into health‑span and aging applications. If successful, this approach could reframe aging as not solely an internal biological clock, but as a process modulated by persistent, low-grade microbial stress, and ultimately, treatable with precision-designed immune molecules.
I also heard echoing ideas about the microbiome’s much bigger role in organism health during the recent Drug Discovery Innovation Program Conference (DDIP 2025) in Barcelona, the event I recently covered for my LinkedIn newsletter. Mainly, in his presentation, Dr. Rafik Fellague-Chebra, Global Medical Director at Novartis Oncology, argued that the microbiome is not a passive bystander in cancer, but an active modulator of treatment outcomes.
Citing recent clinical data, Dr. Fellague-Chebra noted that antibiotic use can reduce the efficacy of immunotherapy by up to 50%, while fecal microbiota transplants (FMTs) from responders can restore treatment response rates. He described this tumor-resident microbial ecosystem, the “oncobiome,” as a key modulator of immune evasion, drug resistance, and pathway-level signaling via microbial metabolites affecting targets like mTOR and AKT.
These insights are in line with findings presented by Maxwell Biosciences at ARDD2024, and together, these perspectives underscore a shift toward a new paradigm where microbiome profiling and modulation could become the future of precision medicine and therapeutic design.