4D Medicine Secures £3.4m to Advance Resorbable Biomaterial Technology
4D Medicine, a spin-out from the Universities of Birmingham and Warwick, has raised £3.4 million ($4.4 million) in a Series A investment round. The funding will enable the completion of pre-clinical testing for its product range and support efforts to obtain FDA clearance for the US market.
(image source: https://4dmedicine.co.uk/)
Investors in this round include Oshen Holdings, DSW Ventures, SFC Capital, Boundary Capital, and several private investors, including leading scientists and surgeons. This brings the company’s total funding to £5 million.
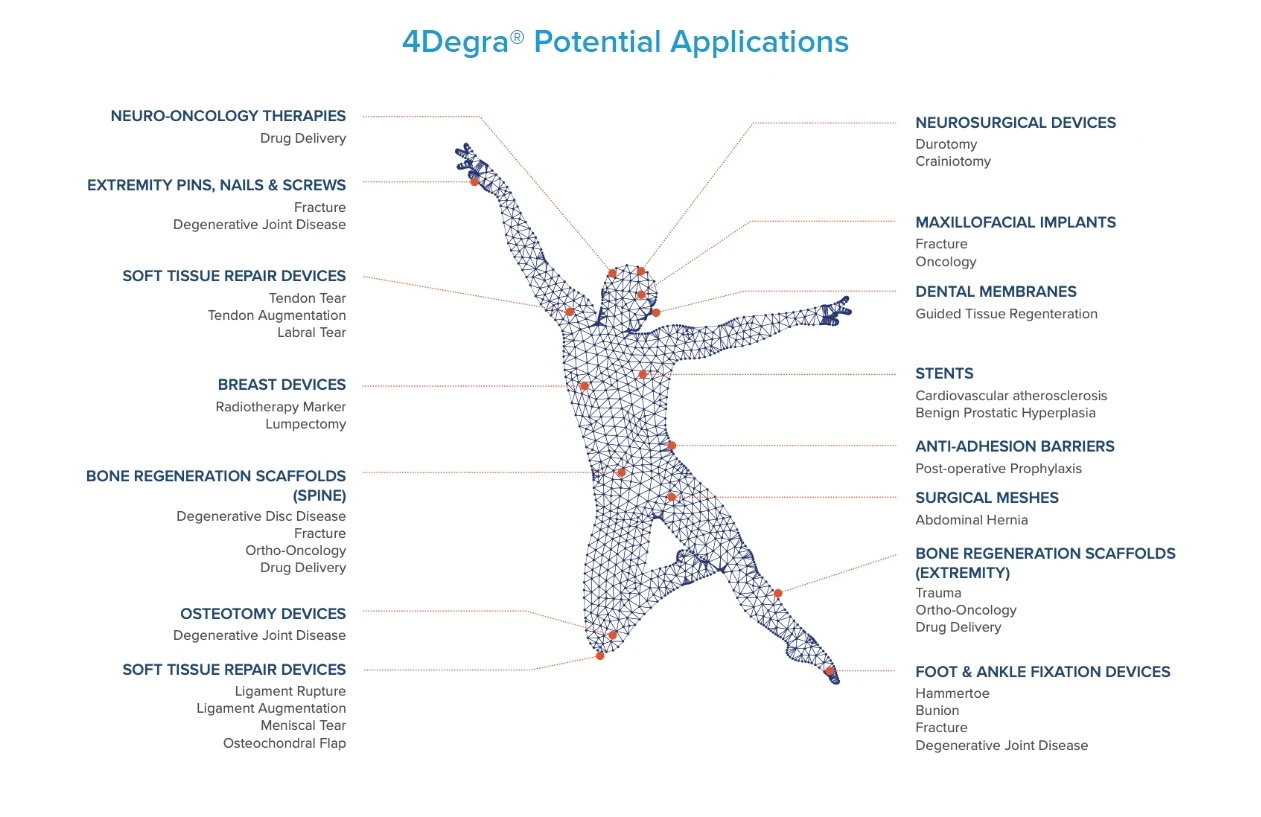
Based in Nottingham, UK, 4D Medicine is developing 4Degra, a resorbable biomaterial designed for 3D printed implants and surgical devices. Unlike existing resorbable biomaterials that degrade quickly and release acidic by-products causing pain and inflammation, 4Degra degrades gradually without harmful by-products. It can be 3D printed into various forms, from soft films to rigid bone scaffolds.
Founded in 2020, 4D Medicine aims to enhance patient outcomes with its innovative biomaterials and medical devices. The company’s facility can produce 1.5% of the global demand for medical-grade resorbable polymers. The market potential for resorbable medical devices exceeds £30 billion.
CEO Philip Smith thanked investors for their support, noting that the funding will help finalize pre-clinical testing and prepare for the orthopedic market. Didier Cowling of Oshen Holdings highlighted 4Degra’s transformative potential in the medical device space, while Doug Quinn of DSW Ventures emphasized the new possibilities opened by 4D’s biomaterial platform.
Legal and advisory services were provided by Addleshaw Goddard, Hill Dickinson, and Weightmans.
4D Medicine Ltd oversees two subsidiaries, 4D Biomaterials Ltd and 4D Medical Devices Ltd, which focus on developing and commercializing resorbable biomaterials and implantable medical devices. Their goal is to improve patient experiences and outcomes through innovative medical technologies.
The company is preparing for a Series B investment round next year as it advances its first medical device product range through the FDA's regulatory processes.
Topics: Tools & Methods