Touchlight and GSK Partner for Enzymatic dbDNA Production in mRNA Manufacturing
Touchlight, a contract development and manufacturing organization (CDMO) specializing in enzymatic DNA production, has entered into a licensing agreement with GSK, a prominent biopharmaceutical company.
This collaboration grants GSK non-exclusive rights to utilize Touchlight’s proprietary enzymatic doggybone DNA (dbDNA™) technology for the production of mRNA-based products.
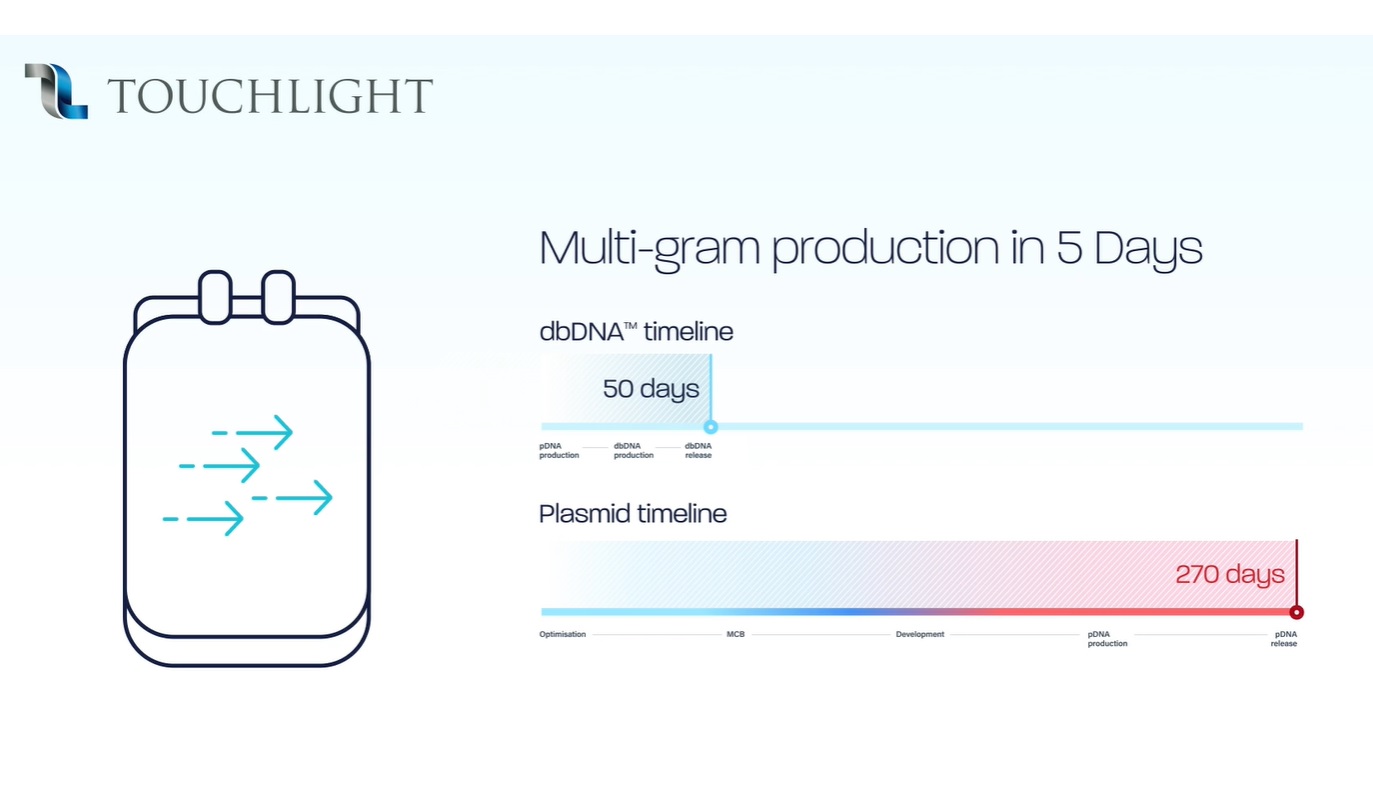
Multi-gram production in 5 days; Image source: Touchlight
Under the terms of the agreement, GSK will leverage Touchlight's dbDNA technology for the rapid and scalable Good Manufacturing Practice (GMP) production of DNA templates essential for mRNA vaccine development. The financial specifics of the deal remain undisclosed; however, it includes an upfront payment, ongoing technology access fees, milestone payments linked to clinical and regulatory achievements, and royalties on GSK’s mRNA products that incorporate Touchlight’s enzymatic dbDNA.
See also: 7 Promising RNA Biotech Companies To Watch in 2024
Touchlight’s dbDNA technology offers a more efficient and scalable alternative to traditional plasmid DNA production by using an enzymatic process to generate high-purity GMP DNA. This is particularly advantageous for mRNA vaccine development, as it allows for the swift and effective production of DNA templates required for mRNA synthesis.
- In 2023, Touchlight inaugurated a state-of-the-art production facility capable of producing multi-kilogram quantities of dbDNA. This facility supports numerous client products in clinical development, including three with accepted Investigational New Drug (IND) or Clinical Trial Application (CTA) status.
- Later in 2024, dbDNA is expected to enter clinical development as an Active Pharmaceutical Ingredient (API) for a therapeutic cancer vaccine, along with multiple other anticipated INDs across various modalities.
Jonny Ohlson, Executive Chair and Founder of Touchlight, emphasized the significance of the partnership, stating:
“GSK is a global leader in vaccination, and we are delighted they have licensed our proprietary enzymatic dbDNA™ technology for the development and production of their mRNA-based products. The adoption of Touchlight’s enzymatic DNA is gathering pace and becoming an important part of the advanced therapy supply chain. Our technology delivers the speed, scalability, and high-quality DNA products that are essential for the next generation of mRNA therapeutics.”
Topics: Manufacturing & Pharma 4.0