Artiva Biotherapeutics Lowers IPO Price, Raises $167 Million for Autoimmune Therapies
Artiva Biotherapeutics, a clinical-stage biotechnology company, has raised $167 million through its initial public offering (IPO). The company offered 13.9 million shares at $12 each, below the anticipated range of $14 to $16, including 5.2 million more shares than initially expected. Artiva Biotherapeutics will list on the Nasdaq under the ticker symbol ARTV.
Read also: 13 Publicly Traded Biotechs Utilizing AI-based Research Platforms (+ 2 Upcoming Public Debuts)
Artiva specializes in off-the-shelf Natural Killer (NK) cell-based therapies for autoimmune diseases and cancers. Unlike traditional therapies that use a patient's own cells, Artiva's candidates are derived from donor cells (allogeneic), pre-manufactured, stored frozen, and ready for immediate use.
Artiva’s lead candidate, AlloNK, is an allogeneic, off-the-shelf, cryopreserved NK cell therapy designed to enhance the antibody-dependent cellular cytotoxicity (ADCC) effect of monoclonal antibodies (mAbs) to drive B-cell depletion. It is in a Phase 1/1b trial for lupus nephritis and an investigator-initiated basket trial for multiple autoimmune conditions expecting initial data in the first half of 2025. Additionally, AlloNK is being evaluated for treating non-Hodgkin lymphoma and in combination with Affimed’s innate cell engager, acimtamig, for treating relapsed/refractory CD30-positive lymphomas.
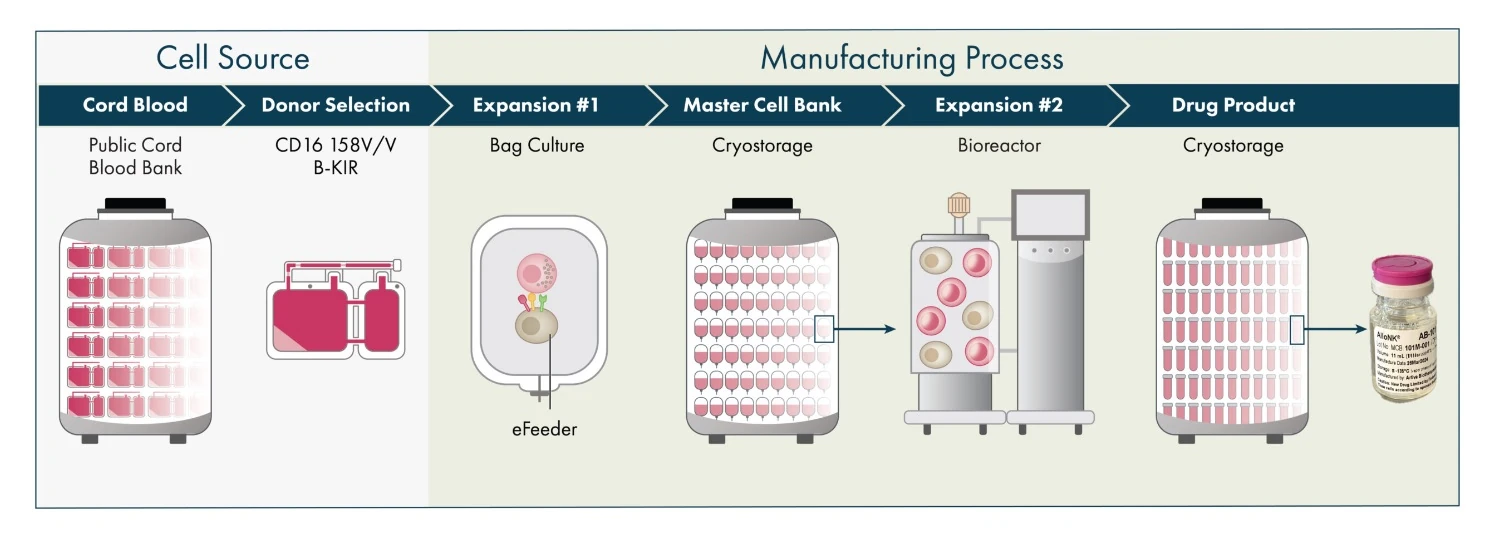
Artiva’s manufacturing platform generates allogeneic NK cells from healthy donor umbilical cord blood (UCB) units selected for the high affinity variant of the CD16 receptor (158 V/V) and a KIR-B haplotype, both markers known to enhance NK cell activity. This process, developed over a decade with strategic partner GC Cell Corporation, does not require genetic modification. Artiva’s facility in San Diego can produce enough AlloNK to treat over 1,000 autoimmune patients annually, with plans to scale up production to meet commercial demand.
(image credit: Artiva)
Cord blood units with preferred characteristics are pre-selected from cord blood banks. Approximately 15% of these units possess the desired attributes. NK cells are isolated, expanded, and cryopreserved to create a master cell bank (MCB). Each MCB unit can yield 80-100+ one billion-cell drug product vials, ensuring batch-to-batch and donor-to-donor consistency. Artiva and GC Cell have produced over 35 clinical batches of AlloNK, totaling thousands of vials.
Artiva’s San Diego headquarters includes a 9,000 square foot cGMP cell production center capable of producing over 6,000 one billion-cell vials annually at the current 50-liter scale. The company is developing a 200-liter commercial-scale process to supply thousands of patients with a cost-effective product.
Topics: Startups & Deals