Light-weight Microscope Captures Large-scale Brain Activity in Freely Moving Mice
Researchers at Rockefeller University have developed a new lightweight microscope capable of capturing large-scale brain activity in mice while they move freely. Published in Nature Biomedical Engineering, the study presents a device weighing 2.5 grams—equivalent to the weight of a US penny—that can provide high-resolution imaging across a broad area of the mouse brain.
Mice are often used as model organisms in neuroscience due to their small size and genetic similarities to humans. However, traditional head-mounted microscopes, which can weigh around 20 grams or about eight US pennies, are too heavy for mice. Previous attempts to reduce the weight of these devices often compromised their field of view, resolution, or depth range, making it difficult to study the entire brain activity effectively.
The research team, led by Alipasha Vaziri, tackled this challenge by rethinking the design of the microscope. Instead of merely making existing technology lighter, they aimed to solve the fundamental problem of mapping points in a three-dimensional sample volume to a two-dimensional camera surface. This approach led to the development of a lens-less system that bypasses the constraints of traditional lens-based designs.
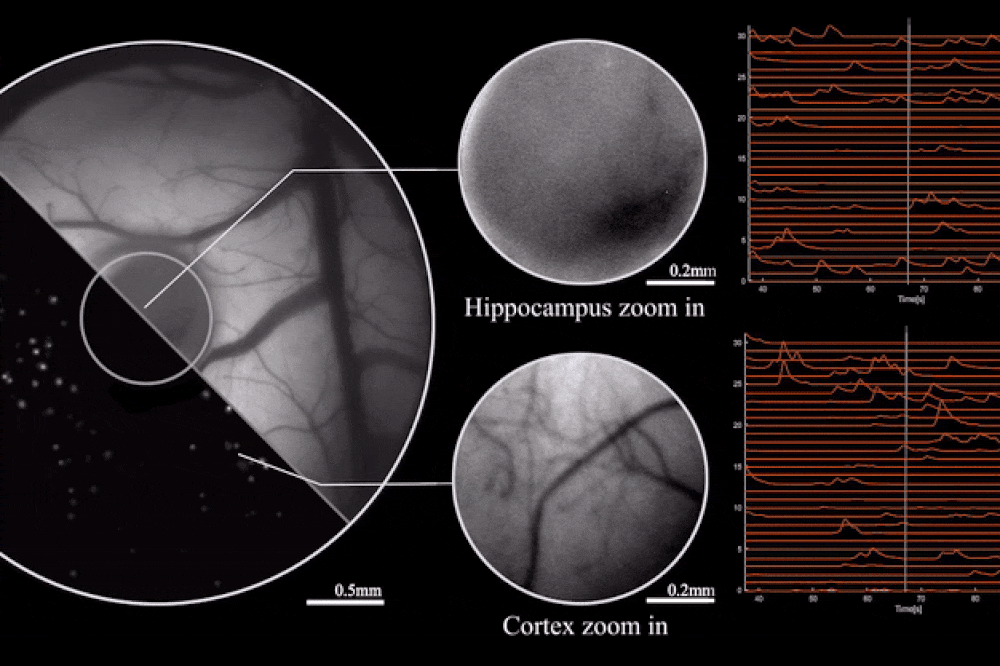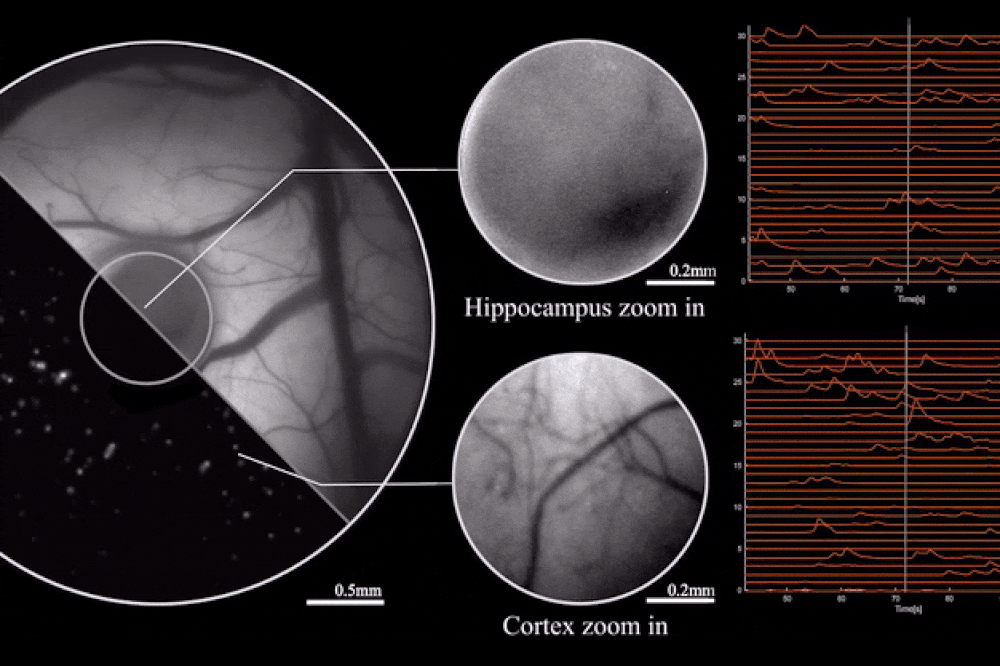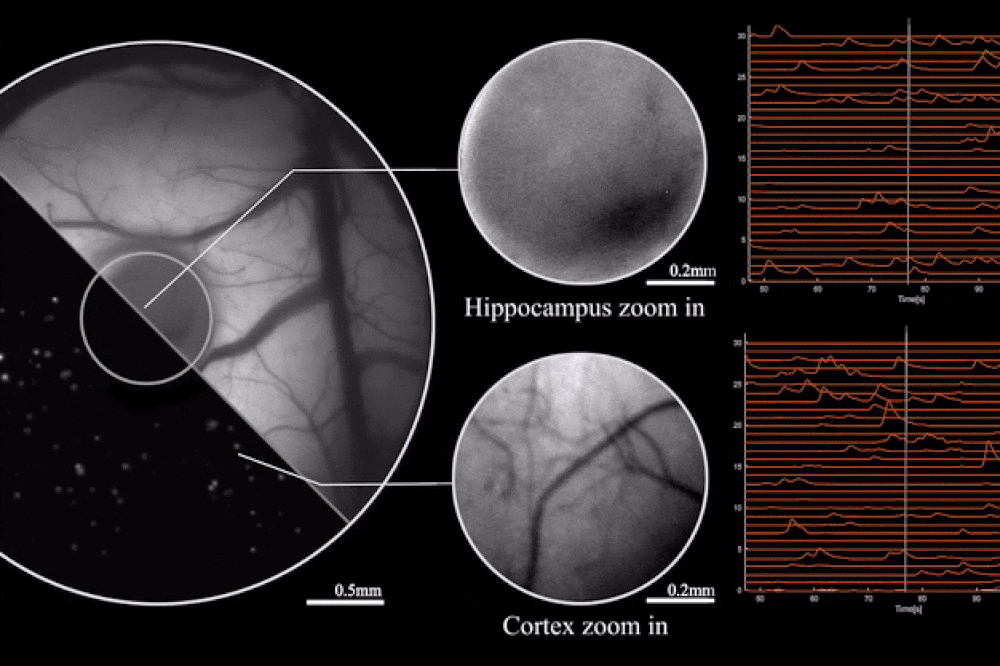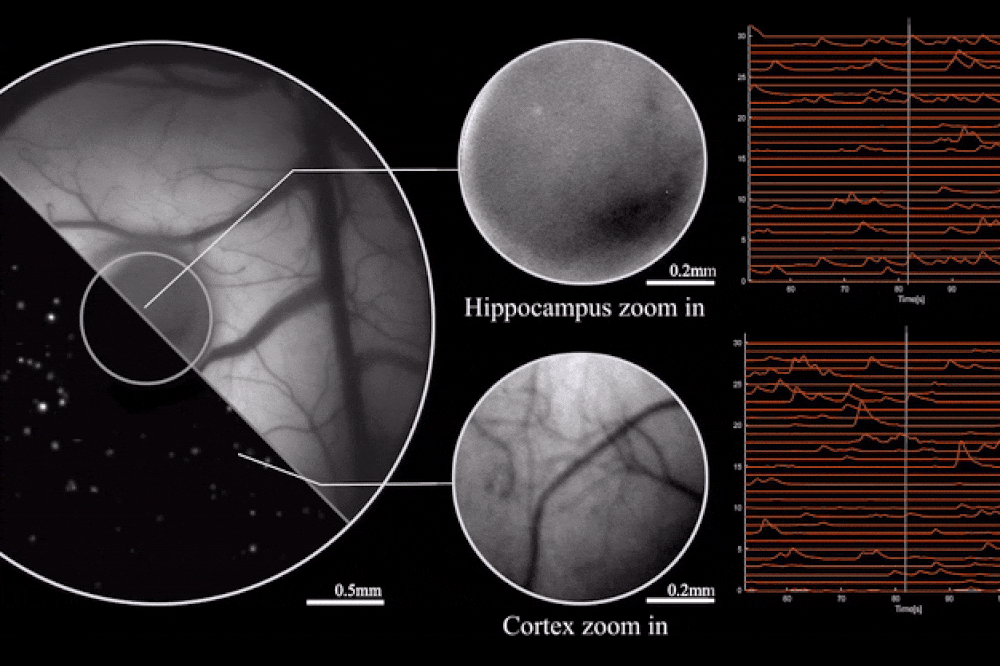
Output from the microscope, as mice engage in natural behaviors. Image credit: Rockefeller University
The key to this innovation is the use of diffractive optical elements (DOEs). Unlike conventional lenses, which rely on a curved surface to manipulate light, DOEs use microstructures to control light waves through diffraction. This allows for precise mapping of the scene to the sensor without the need for heavy lens components. The result is a microscope that can record brain activity with a 3.6 x 3.6 mm² field of view, 4 μm lateral resolution, 300 μm depth of field, and a recording speed of 16 volumes per second.
The mini microscope's design makes it accessible and affordable, as most of its parts can be 3D printed or obtained from consumer-grade camera sensors. Vaziri pointed out that interested labs could build these microscopes at low cost.
Future versions of the microscope may include wireless data transmission. The current model uses cables, which are manageable for single mice but could become problematic when observing multiple mice interacting. Enhancements may also allow for the observation of deeper brain regions.
While the system has some limitations compared to larger microscopes, Vaziri emphasizes the importance of innovative thinking in overcoming these constraints. This lightweight, high-resolution microscope represents a practical tool for advancing the study of brain activity and behavior in mice.
The study also highlights the optimization of the miniaturized head-mounted fluorescent mesoscope for calcium imaging at single-neuron resolution. The device is stable against motion-induced artifacts and can record neuronal activity at up to 16 Hz. This capability was demonstrated during experiments involving socially interacting mice and fear-conditioning tests, providing insights into neurovascular coupling across multiple cortical regions.
Topics: Tools & Methods