Santa Ana Bio Secures $168 Million to Propel Precision Medicine for Inflammatory Diseases
Santa Ana Bio, a precision immunology company focused on developing targeted therapies for autoimmune and inflammatory diseases, has officially launched with $168 million in combined Series A and B funding. The initial Series A round was spearheaded by Versant Ventures with contributions from TPG’s Life Science Innovations fund and GV. The subsequent Series B round, totaling $125 million, was led by GV and included investments from Access Biotechnology, Andreessen Horowitz (a16z) Bio + Health, and RTW.
Santa Ana Bio's approach diverges from traditional biologics for autoimmune diseases, which typically target cytokines—the messengers rather than the causative agents of disease—and do not differentiate between diseased and healthy tissue. Instead, Santa Ana employs advanced proteomic, transcriptomic, and genomic techniques to identify disease-specific subpopulations and targets on disease-causing cell types, sparing healthy cells. This strategy aims to improve patient outcomes by addressing issues like patient heterogeneity, treatment durability, and safety.
"Precision medicines offer the ability to target defined cell types and pathways to disrupt the pathogenic biology driving disease," explained Peter Emtage, Ph.D., CEO and board member of Santa Ana Bio, who is also a venture partner at Versant. "By identifying pathogenic cell types and pathways, and leveraging our deep protein engineering capabilities, including monoclonal, bi-specific antibodies and ADCs, we aim to expand the reach of biologics to patients across numerous inflammatory diseases."
Dr. Emtage brings over two decades of drug development experience in autoimmunity, inflammation, oncology, and infectious diseases to his role. His previous positions include global head of cell therapy research at Kite Pharma, a Gilead company, and CSO at Cell Design Labs, which was acquired by Gilead. He also served as VP of immune-mediated therapy at MedImmune.
Jerel Davis, Ph.D., managing director at Versant and board chair of Santa Ana, emphasized the company’s approach:
"Each of Santa Ana’s programs builds upon clinical precedent, yet provides important differentiation from past approaches. Given the breadth of these programs and pipeline, Santa Ana clearly has optionality to advance these promising internal programs and to pursue partnerships."
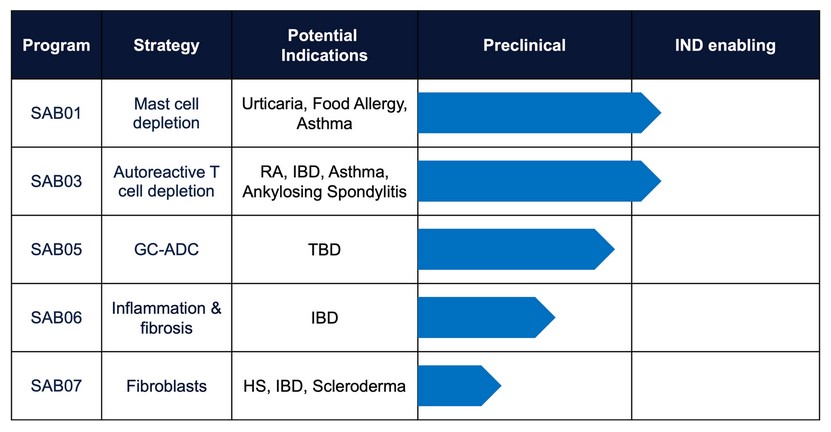
Santa Ana Bio pipeline; Image credit: Santa Ana Bio
The company has swiftly progressed since its inception in 2022, with three programs set to enter clinical trials next year. These precision immunology therapies target causal cells and are designed to outperform existing treatments.
- SAB01 targets c-Kit on mast cells involved in allergic diseases. To mitigate the toxicity historically associated with this target, Santa Ana developed a bi-specific antibody that conditionally blocks c-Kit only in the presence of an undisclosed anchor target restricted to mast cells. SAB01 has shown conditional activity against mast cells in vitro and in vivo and is slated for Phase I testing in chronic inducible urticaria next year.
- SAB03 is designed to agonize PD-1, a validated target in rheumatoid arthritis, addressing all antigen-reactive T cells, including high-, medium-, and low-expressing PD-1 pathogenic T cells. This candidate is also set for Phase I testing next year for various severe inflammatory conditions.
- The SAB05 ADC program utilizes a novel antibody-glucocorticoid conjugate technology, allowing Santa Ana to leverage the therapeutic efficacy of glucocorticoids while avoiding the associated collateral damage.
Topics: Startups & Deals