Lantern Pharma Receives Japanese Patent for Oncology Drug Candidate LP-284
Lantern Pharma Inc., an AI-driven company focused on oncology drug development, has announced that the Japan Patent Office (JPO) has granted a patent for their drug candidate LP-284, a novel small molecule agent that damages DNA in cancer cells leading to their death.
This patent, identified by application number 2021-513267 and registration number 7489966, covers the composition of matter for LP-284, a compound developed as a potential treatment for relapsed or refractory non-Hodgkin's lymphoma and specific types of sarcomas.
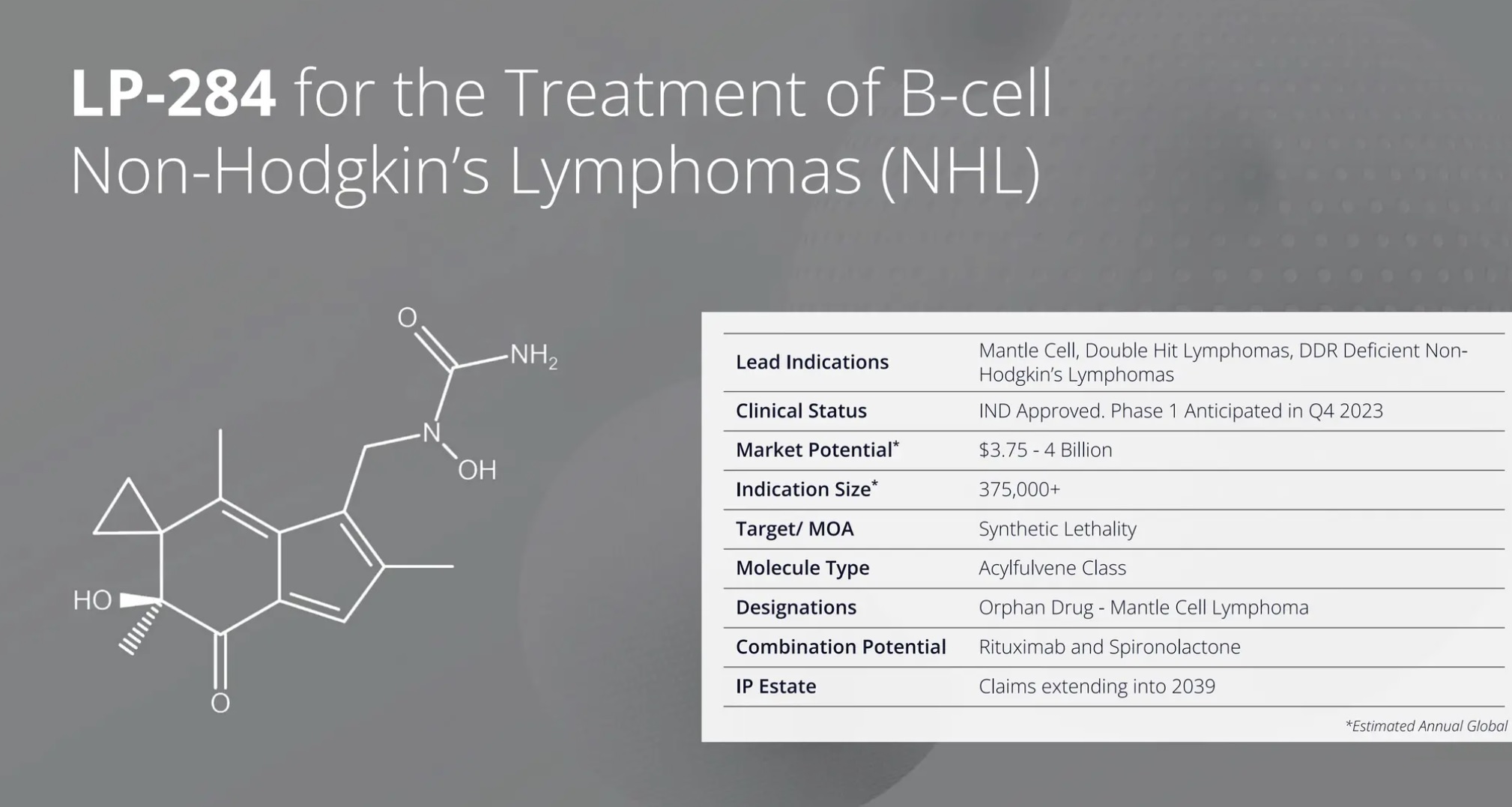
LP-284 datasheet
LP-284 has reached Phase 1 clinical trials, demonstrating progress through Lantern Pharma's RADR platform, an AI and machine learning system designed to accelerate drug discovery and development. RADR utilizes over 60 billion oncology-focused data points to identify promising drug candidates and potential patient populations. LP-284 is the third molecule to enter clinical trials with support from RADR, underscoring the platform's effectiveness in expediting the drug development process.
Japan is the third country to grant a composition of matter patent for LP-284, following similar approvals in the United States and other regions. This patent enhances the global intellectual property protection for LP-284 and supports potential future clinical collaborations and geographic partnerships.
Lantern Pharma projects that LP-284 could benefit 40,000 to 80,000 patients annually, with a global market potential estimated at $4 billion USD.
See also: Will Biologics Surpass Small Molecules In The Pharmaceutical Race?
In the United States, LP-284 has been granted Orphan Drug Designation by the FDA for the treatment of high-grade B-cell lymphomas and mantle cell lymphoma (MCL), which emphasizes the drug's potential in addressing unmet medical needs in these indications. In 2023, the FDA approved an investigational new drug (IND) application for LP-284, leading to the initiation of a first-in-human Phase 1 clinical trial. The trial, currently ongoing, includes patients with various subtypes of non-Hodgkin's lymphoma and select solid tumors and sarcomas.
MCL, a subtype of non-Hodgkin's lymphoma, affects approximately 5,800 individuals annually in Japan and around 9,000 patients combined in the U.S. and Europe. LP-284 offers a potential therapeutic option for patients who experience relapse after initial treatment, addressing a critical need for improved treatment strategies in this patient population.
Topics: Clinical Trials