Sponsored by Trialize
Revolutionizing Clinical Trials: How AI and Automation Reduced Study Build Time from Months to Days
In the realm of clinical trials, efficient data management and monitoring are crucial yet often hampered by outdated and time-consuming practices. This case study delves into the implementation of Trialize, an artificial intelligence (AI)-powered platform, by a contract research organization (CRO).
It highlights how Trialize's innovative approach to automating study builds and leveraging AI for data analysis significantly reduced the time from protocol to trial, transforming the process from a traditionally months-long undertaking into a matter of days.
This narrative aims to showcase the tangible impacts of AI and automation on clinical trial efficiency, offering insights into the platform's capabilities and the practical benefits realized by the CRO partner.
Overview of Trialize
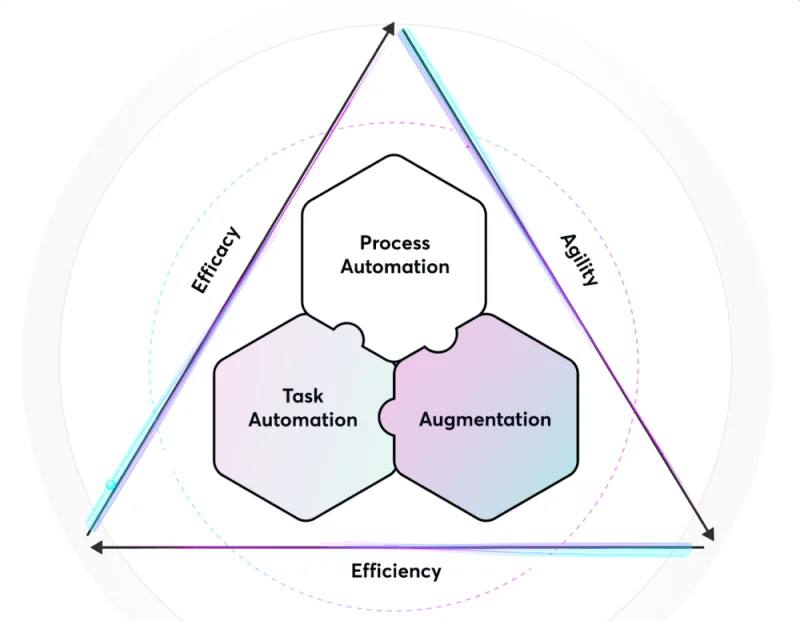
Trialize is an AI-enhanced platform designed to streamline the clinical trial process. It introduces a significant paradigm shift in how study builds are managed and executed, addressing several inefficiencies inherent in traditional clinical trial methodologies.
When inputting the study protocol, Trialize's AI analyses it to extract essential information, such as visit schedule, relevant forms, eCOA scales, and even drafts initial Informed Consent Forms (ICFs).
What sets Trialize apart is the environment in which its AI operates. The AI resides in a controlled, isolated setting, specific to the CRO’s instance. This isolation ensures data integrity and security while allowing the AI system to evolve in sync with the CRO’s expanding experience and dataset.
As more studies are conducted through Trialize, the AI's ability to rapidly produce new study builds enhances, effectively learning and adapting to the organization’s specific needs and patterns.
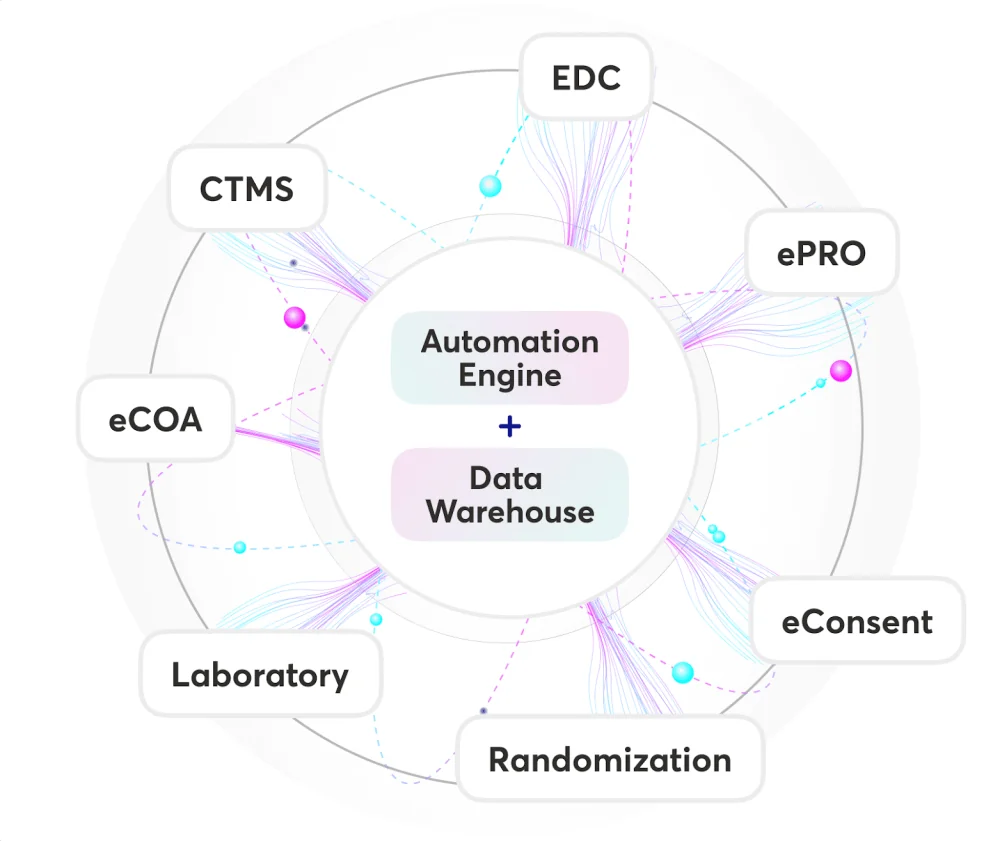
Trialize’s architecture offers a comprehensive, integrated solution covering all aspects of clinical trial management – from Electronic Data Capture (EDC) and Electronic Patient-Reported Outcomes (ePRO) to TeleVisits, eConsents, and Analytics. Its interface is designed to be intuitive and user-friendly, drastically reducing the learning curve for study teams.
The platform also eliminates the need for extensive training, as most users can familiarize themselves with the system's study build and data entry processes within a few hours.
The backend of Trialize is underpinned by a sophisticated Data Warehouse and Automation engine. This combination provides unparalleled flexibility in managing data without sacrificing capability or control.
Users can access and manipulate data effortlessly, and the platform’s low-to-no-code interface allows for the easy implementation of complex workflows and clinical trial processes.
Case Example: Streamlining Complex Workflows in Clinical Trials with Trialize
Project Overview and Challenge
A Contract Research Organization (CRO) encountered significant challenges in managing the complexity of clinical trial processes. The primary issue was the time-intensive nature of creating production-ready study builds, along with the difficulty in integrating various systems and managing complex data workflows.
Implementation of Trialize
In addressing these challenges, the CRO implemented Trialize in two of its clinical trials. Trialize is built on top of advanced Data Warehouse and Automation engines, enabling the CRO to simplify complex processes significantly.
Detailed Execution and System Capabilities
The CRO utilized Trialize's data object model, which provided flexibility without sacrificing capabilities. This model made every data piece rapidly accessible and allowed full control over all events related to the data. The CRO particularly benefited from the platform's automation engine, which offered an intuitive, low-to-no-code interface. This enabled the CRO to implement complex workflows and business processes associated with clinical trials without relying on programmers or external consultants.
A notable achievement with Trialize was the development of a 28-step BPMN-type workflow that integrated with two external systems, including Labs and PACS Imaging, which was completed in just 5 hours by a single specialist. This efficiency underscored Trialize's ability to act as a central data and process hub, efficiently mediating data and event flows within the clinical trial.
Advanced Monitoring and Real-Time Analytics
Additionally, Trialize featured an advanced analytics module, offering comprehensive exploration and visualization capabilities. This module came equipped with pre-made dashboards for central and risk-based monitoring, comparable in functionality to PowerBI. This allowed for significant flexibility and control over which indicators were most relevant for specific cases. Due to its tight integration with other modules, like EDC, Randomization, and Coder, Trialize enabled a deeper level of insight by allowing the integration of external data.
One of the most impactful aspects was the ability to share dashboards directly with sponsors, providing real-time synchronization and insights. This feature eliminated the need to produce and share reports via email, ensuring that sponsors had constant access to the latest data and analytics.
Results and Impact
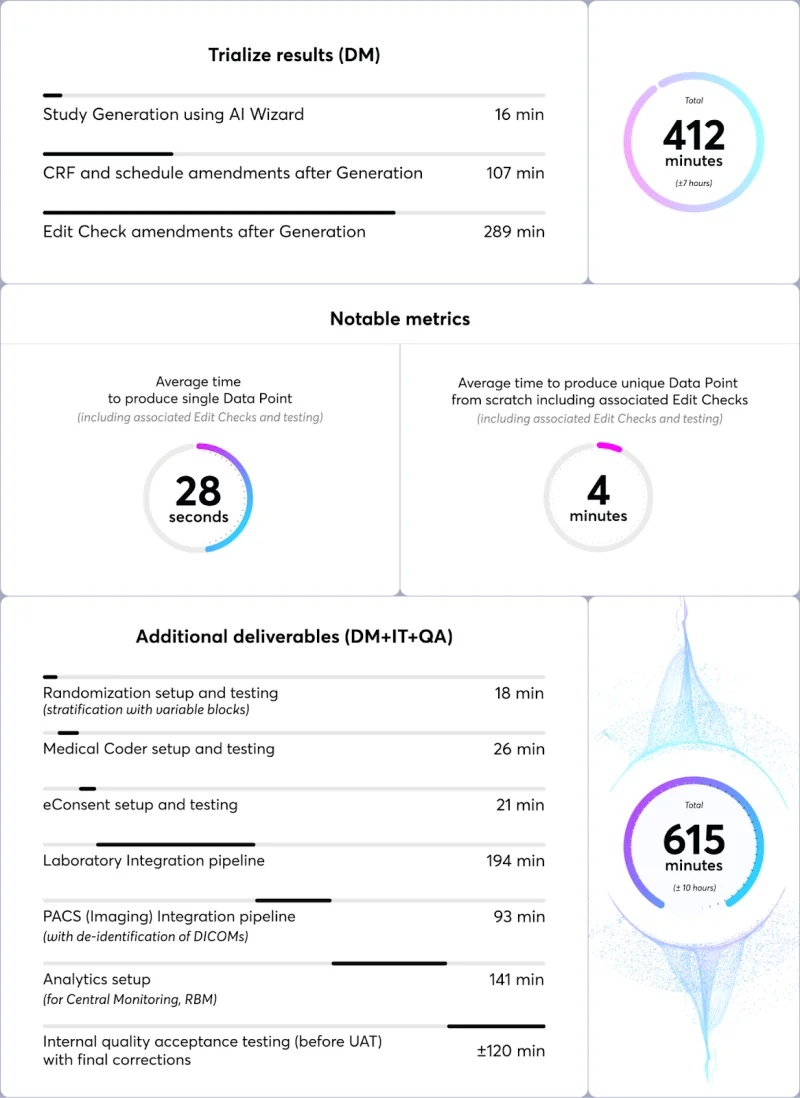
An equivalent setup using a market-leading vendor, implemented by certified, trained specialists, would take approximately four or more weeks.
Additionally, it would require qualified software developers to create custom data integration bridges for Labs, Imaging, and BI (e.g., PowerBI), as well as data analysts to configure visualization and reporting packages, specifically tailored for the study. In contrast, with Trialize it’s possible to go live in days, not months.
By implementing Trialize, the CRO achieved a significant reduction in the time required to start up, transforming a process that once took months into a matter of days.
The use of Trialize's sophisticated data management and workflow automation tools not only streamlined complex processes but also enhanced data integrity and compliance. The advanced analytics capabilities provided the CRO and their sponsors with real-time insights, greatly improving decision-making efficiency and overall trial management.