The Time for Breakthroughs in Antibiotic Discovery
Since a revolutionary discovery of penicillin in 1928 by Scottish bacteriologist and Nobel laureate Alexander Fleming, numerous inventions of new antibiotic classes followed. A cascade of discoveries over decades pushed the limits of medicine in its ability to fight deadly infections, those that used to kill millions of people in the previous centuries.
A "silent pandemic"
According to the statistics from The Center of Disease Control and Prevention in the United States (the CDC), around 2.8 million people get infected by antibiotic-resistant illnesses in America each year, of whom around 35,000 die each year because antibiotics simply don’t work as they used to in the not-so-distant past. According to another source, drug-resistant diseases kill around 700,000 people each year globally, but a United Nations interagency group on antimicrobial resistance estimates that this could swell to 10 million a year by 2050 if no action is taken. This is more than the number of people who currently die from cancer worldwide every year.
A recent report by Massachusetts Department of Public Health stated that a disease as old and well known as Gonorrhea is developing resistance to the last antibiotics that are efficient against it, and no new cures are so far available to substitute the existing ones.
We have been successfully using antibiotics in so many cases and for such a long time that started taking this powerful tool of modern medicine for granted. Meanwhile, the warning signs making headlines informing the public about emerging “superbugs” able to withstand antibiotics due to developed multidrug resistance and thereby becoming a deadly risk.
The dangerous infection MRSA (methicillin-resistant Staphylococcus aureus) was reported spreading in hospitals and healthcare facilities, while uncontrolled overuse of antibiotics in livestock farming and in some medical practises led to the emergence of new resistant strains of bacteria such as Salmonella and E. coli. These are just examples, the list can go on.
As widely used therapeutics such as tetracycline, erythromycin and vancomycin lost much of their effectiveness against bacterial infections over the years, the antibiotics Colistin and Carbapenem are considered the big guns — a last line of defense when no other antibiotics are working, for example in the case of multidrug-resistant Gram-negative infections.
Colistin structure
Such medications are called drugs of last resort and they usually possess drastic side effects, as in the case of Colistin, being toxic to the human kidney; still, they are the last hope for some desperate patients. Now even this last weapon against malicious bacteria is becoming obsolete, as it was reported in recent years, mcr-1, a gene which confers resistance to Colistin, has been found in E.coli from over 30 countries, including the cases of resistant bacteria isolated in China and in the United States, and in other regions.
The same story happens in the case of Carbapenem when the gene blaNDM-5 renders bacteria resistant to its action. In 2012, the CDC identified Carbapenem-resistant infections in about 4 percent of US hospitals. Here is a recent research (March 2022) explaining statistics and dynamics of spread of the gene blaNDM-5, and its contribution to the rise of global antibiotics resistance.
The problem goes beyond treating infections. According to Army Col. Emil Lesho, director of the Defense Department’s Multidrug-resistant Organism Repository and Surveillance Network in the United States, the growth of bacterial resistance puts humanity at risk of losing access to modern medical “miracles”, such as medical surgeries, joint replacements, organ transplantation, cancer chemotherapies etc. These treatments can not be safely performed without antibiotics because it is almost impossible to avoid bacteria penetrating the body during severe medical interventions.
Finally, the viral COVID-19 pandemic contributed indirectly, but severely, to the deterioration of the situation with bacterial resistance. Due to the focus on COVID-19 in 2020, CDC reported that progress against antimicrobial resistance has been lost as noted in the CDC's 2022 Special Report on the impact of the pandemic. Antimicrobial resistance could spread in the acute care setting as a result of the COVID-19 pandemic's increased use of antibiotics. In order to prevent potential bacterial infections, antibiotics have been used frequently in hospitals with COVID-19 patients. Up to 70% of COVID-19 patients are treated with antibiotics, either as inpatients or outpatients. Virens cannot be eliminated by antibiotics. But doctors frequently have to give antibiotics to COVID-19 hospital patients who have a confirmed or strong suspicion of a bacterial coinfection or superinfection.
Why has the crisis begun?
The history of antibiotics research is the history of a constant race between drug discovery researchers and ever-evolving natural enemy - bacterial infections. Since the discovery of early antibiotics, it was noticed that bacteria would evolve rather quickly adjusting to the new environment and developing resistance to external molecules. Once resistance was developed rendering the existing antibacterial drug useless, a new type of antibiotics was needed to save the day.
Drug discovery industry used to do a good job at keeping up with natural bacterial threats and numerous new classes of antibiotics were discovered in the mid of the last century. However, the progress slowed down and almost vanished by the 2000s. In fact, researchers haven’t identified a new class of antibiotic medication since 1987, while bacteria kept developing resistance to the previously invented drugs. So why has innovation slowed so dramatically?

image credit: e-Bug, operated by the UK Health Security Agency
A major reason of the substantial decline of novel antibiotics approvals in reacent three decades is the economics of drug development of antimicrobials. Pharmaceutical companies spend hundred of millions of dollars on drug discovery research, multi-step clinical trials and the FDA approval process, thus, the result has to pay back very well, for a long time and with minimum business risks.
It stimulates companies concentrate on therapeutic areas where there are chances to develop blockbuster drugs. An ideal candidate would be demanded by millions of people on regular bases for a very long period of time, like pills to keep cholesterol in check, or normalize blood pressure or maintain sexual life active. A very crowded space is also oncology research and drug discovery.
But antibiotics are among the worst candidates, businesswise (at least, until recently...keep reading).
They have strict prescription limits and should only be used for about a week or longer. They are relatively cheap, for example, compared to anti-cancer drugs, and worst of all, they compete in a field of inexpensive generics making it hard to gain profits. On top of that, antibiotics can become obsolete at any time due to developed bacterial resistance and this is a substantial risk for business.
As Dr. Anthony Fauci, former director of the National Institute for Allergies and Infectious Diseases in the United States, once stated, without government policies encouraging investment in antibiotics development, “there’s very little incentive” for pharmaceutical companies to go this way".
Furthermore, according to a World Health Organization report released in 2021, none of the 43 antibiotics currently in development "adequately address the problem of drug resistance" in the bacteria thought to be the most harmful to public health.
The lost (and reviving) art of antibiotics discovery
However, besides purely business-related reasons, there are other reasons why we have not seen major innovations in the antibiotics space ever since the 'golden age' of antibiotics discovery. Such reasons are briliantly summarized in a recent paper: "Brief Overview of Approaches and Challenges in New Antibiotic Development: A Focus On Drug Repurposing", such as collapse of the Waksman Platform and shift from soil-based discovery to other strategies (which proved to be largely unsuccessful). It was also a shift from phenotypic discovery of antibiotics (in vitro growth inhibition assays) to target-based discovery and HTS, which was all widely regarded as a progressive move (and it proved so in many therapeutic areas), but proved to be a failure specifically in the antibiotics space. An increasing focus on medicinal chemistry efforts in contrast to reliance on natural sources of inspiration, an industry bias towards Rule-of-5 molecules were also possible contributors in the overall decreasing productivity in terms of being able to discover noval classes of antibiotics.
According to Dr Chris De Savi, VP of Drug Discovery at Kymera Therapeutics, only 17 new systemic antibiotics and 1 related biologic have been approved by the FDA since 2010. Among these drugs, 14 were approved for common bacterial infections, 1 was approved for Clostridioides difficile infection (CDI), 1 was licensed to prevent CDI recurrence, and 2 were approved for drug-resistant tuberculosis. Unfortunately, the majority of these new drugs represent modifications to existing chemical structures rather than new drug classes.
"In contrast to the Gram-positive field, where a variety of treatment options are available, the resistance situation in infections caused by Gram-negative bacteria is dramatic. Pan-resistant Gram-negative enterobacteriaceae or non-fermenters have only increased lately." Dr. Chris De Savi further notes.
Reviving the field of antibiotics discovery
No matter the reasons of the antbiotics discovery crisis over the last several decades, the situation with the growing threat of antibiotics resistance keeps worsening, and the industry, as well as governments, are trying to change the "anti-trend" towards more productive realms.
In 2012, the United States administration introduced the Generating Antibiotics Initiatives Now (GAIN) Act, which was designed to provide incentives for pharma businesses and investors to play the game of antibiotics discovery. The Act extended the exclusivity of new antibiotics by five years over any existing exclusivity, such as the ongoing patent protection, Hatch-Waxman, orphan drug or pediatric exclusivity. It gave extra-time for pharmaceutical companies to market antibiotics without the need to compete with generics. In addition, all new antibiotics that fall under the GAIN provisions receive fast track and priority review status which substantially accelerates the approval process with FDA.
Recently introduced The Pioneering Antimicrobial Subscriptions to End Upsurging Resistance (PASTEUR) Act would establish a subscription-style payment model whereby the federal government would pay upfront for access to FDA-approved antibiotics that target drug-resistant pathogens and address urgent, unmet medical needs. The purpose of the bill, which would decouple company profits from the amount of antibiotics sold, is to assist in resolving market issues that have caused many pharmaceutical companies to stop developing antibiotics and contributed to the lack of new, innovative antibiotics in the pipeline.
A new wave of players and innovations
While antibiotics drug discovery remains notoriously difficultt area for entrepreneurship, new players emerge in this area every year. In 2020, the Boston-based AMR Action Fund, supported by pharmaceutical companies and organizations like Wellcome Trust, began funding early-stage research and clinical trials of antimicrobial drugs at smaller biotechnology companies. The fund dedicated $1 billion to invest in antibiotics companies, and has several antibiotics companies in its portfolio, including Bioversys, a clinical stage Swiss pharmaceutical company focusing on research and development of small molecules acting on novel bacterial targets with applications in antimicrobial resistance (AMR) and targeted microbiome modulation. The company's pipeline addresses nosocomial infections of Acinetobacter baumannii (BV100, Phase 2 ready), and tuberculosis (BVL-GSK098, Phase 2 ready) in collaboration with GSK and a consortium of the University of Lille.
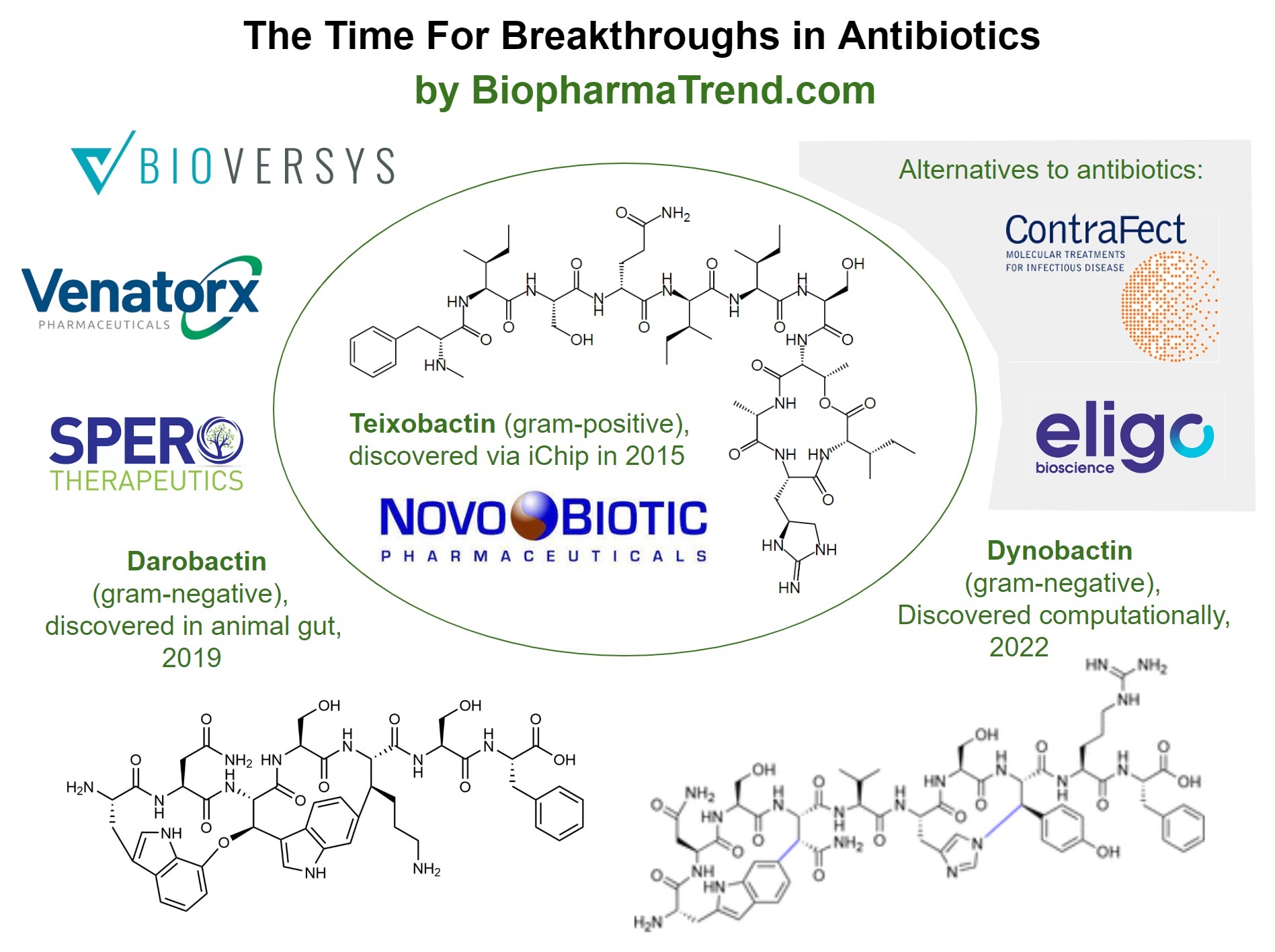
AMR Action Fund's portfolio also includes Venatorx Pharmaceuticals is a private company that is focused on the discovery and development of novel anti-infectives to treat multi-drug-resistant (MDR) bacterial infections and hard-to-treat viral infections. Both of Venatorx's lead antibacterial clinical-stage programs, cefepime-taniborbactam and ceftibuten/VNRX-7145, are combinations of broad-spectrum beta-lactam / beta-lactamase inhibitors. Additionally, Phase 1 clinical development has begun on the first Venatorx antiviral compound, VNRX-9945, an inhibitor of the Hepatitis B virus. Penicillin Binding Protein (PBP) inhibitors are a novel class of non-beta-lactam antibiotics that the company is developing as part of its discovery-stage programs. These inhibitors have the potential to overcome the 70+ years of resistance to penicillin and usher in a new era of antibacterial therapeutics.
Founded in 2013 and traded publicly on Nasdaq since 2017, Spero Therapeutics is a multi-asset, clinical-stage biopharmaceutical company focused on identifying, developing and commercializing novel treatments for bacterial infections, including multi-drug resistant, or MDR, bacterial infections, and rare diseases. SPR720 is an oral antimicrobial agent in development by Spero Therapeutics for the treatment of nontuberculous mycobacterial (NTM) pulmonary disease, a rare orphan disease. The IV administration of SPR206 is being researched as a potential new way to treat multidrug-resistant Gram-negative bacterial infections in hospitals. To treat cUTIs, including pyelonephritis, caused by specific microorganisms in adult patients with few other oral treatment options, tebipenem HBr was developed as the first broad-spectrum oral carbapenem-class antibiotic.
Founded in 2015, Entasis Therapeutics, a spinout of AstraZeneca, raised almost $200 million in multiple rounds and managed to build a robust pipeline consisting of clinical and pre-clinical pathogen-targeted small-molecule antibacterials for the treatment of multidrug-resistant Gram-negative bacteria.The Entasis's drug discovery platform combines the use of genetics tools, molecular dynamics simulations and modeling to enable exploration of novel therapies in a highly directed, focused manner.
Discovery of Teixobactin using iChip
In 2015, a group of scientists led by Dr. Kim Lewis, Director of the Antimicrobial Discovery Center at Northeastern University, reported a new antibiotic, teixobactin, able of killing several types of bacteria, including antibiotic-resistant strains of tuberculosis and staphylococcus (MRSA infections) without detectable resistance developing over time. Teixobactin is a small molecule antibiotic of a new classcapable of destroying 'drug resistant' bacteria.
What is even more groundbreaking is a way by which the compound has been found. Researchers developed an innovative reinvention of an old technique used in the past for many of the antibiotic discoveries of the mid-twenties century - from soil samples.
Combing soil samples for microbes producing their own antibiotic compounds to kill competing bacteria were once a powerful technique by which many of the early antibiotics were found. However, when all the compounds that were easiest to be found this way and cultivate in a laboratory were identified, innovations largely dried up.
Dr. Lewis and his colleague at Northeastern, Dr. Slava Epstein, revived soil mining approach by inventing the iChip. This device allows growing in a lab bacteria that were impossible to cultivate by previous techniques. In this new approach, soil samples are placed between the iChip membranes and the device is buried back in the ground where bacteria can get nutrients from the soil in a natural way. Once colonies grow within the iChip, they are transferred back to the lab for discovering antibiotics.
Overall, about 50 000 strains of uncultured bacteria have been grown using the iChip and 25 new promising antibiotic compounds identified, including teixobactin. The new invention has a potential to solve the biggest issue of antibiotics research - the lack of drug candidates. Systems like the iChip enable exploration of large numbers of compounds with promising antibacterial properties which is the key to fighting the resistance phenomenon.
Northeastern University licensed the patent on the iChip technology and any compounds produced to an early-stage biotech company NovoBiotic Pharmaceuticals (Cambridge, MA), founded by the researchers. The research is curretly ongoing to follow up the teixobactin discovery and translate it into clinical practise. For instance, in August 2022, a major nature article was published, where an international coalition of laboratories including NovoBiotic report that teixobactin kills bacteria through a two-pronged attack on the cell envelope.
Discovery of Darobactin from animal microbiome
In 2019, a team of researchers led by Dr. Kim Lewis announced their discovery of Darobactin, which can kill resistant gram-negative bacteria. The substance was discovered by Yu Imai, a postdoctoral research associate in Lewis' lab, from Photorhabdus bacteria that reside inside the gut of a nematode, a tiny parasitic worm found in soil. Lewis claims that this is the first time an antibiotic with potential for human use has been discovered in the animal microbiome.
Darobactin measures 965 Daltons, which is unusually large for an antibiotic. That made it unclear how darobactin functions since it is too large to pass through gram-negative bacterial membranes. The scientists repeatedly exposed E. coli to the substance until bacterial resistance developed in order to find out. These bacteria had mutations in a gene that produces the BamA molecule, which covers the bacteria's outer membrane. It was a very interesting finding, an outer-membrane protein.
According to Karen Bush, a biochemist at Indiana University, not all gram-negative bacteria are inhibited by the substance at low concentrations. According to her, Darobactin may serve as a novel scaffold that drug chemists can modify to create a more widely effective medication.
Discovery of Dynobactin via computational screening
Another recent success aiming at tackling Gram-negative bacteria is the discovery of Dynobactin in 2022. It is a new antibiotic that was discovered and its mechanism of action identified by an international team of researchers at the University of Basel in Switzerland. The study was conducted by the researchers in collaboration with Professor Sebastian Hiller from the University of Basel's Biozentrum as part of the NCCR "AntiResist" initiative; results published in Nature Microbiology.
The fact that many bacteria produce antibiotic peptides to compete with one another was exploited by the researchers. Further to that, unlike natural substances, these peptides are encoded in the bacterial genome. It was found that the genes for such peptide antibiotics share a characteristic feature, and using this feature, the computer thoroughly screened every molecule in the genome of the bacteria that produce these peptides, and eventually landed on the discovery of Dynobactin.
The bacterial membrane protein BamA, which is crucial for the development and upkeep of the outer-protective bacterial envelope, is blocked by this peptide. Despite having very few chemical similarities to the previously identified Darobactin, Dynobactin nonetheless targets the same area of the bacterial surface, which was an unexpected discovery.
Alternative first-in-class modalities to tackle antimicrobial resistance
Antibiotics is the most widely adopted way to fight bacterial infections. However, it is not the only modality available in the arsenal of medicine. A number of companies are advancing alternative technologies to tackle hard-to-treat diseases.
For example, Eligo Bioscience is deploying in vivo gene editing technologies, based on CRISPR, against microbiome targets to address diseases with high unmet needs. Eligo was founded by scientists from The Rockefeller University and from MIT. Eligo was named a Technology Pioneer by the World Economic Forum and has received venture capital funding from Khosla Ventures and Seventure Partners, and non-dilutive funding from GlaxoSmithKline, the European Commission, CARB-X, and Bpifrance.
In October 2022, FDA has granted Orphan Drug Designation and Rare Pediatric Disease designation for Eligo's oral drug candidate EB003, for the treatment of Shiga-toxin producing bacterial infection as it relates to the prevention of hemolytic uremic syndrome (HUS).
Another interesting company is ContraFect Corporation, which develops a new class of antimicrobial peptides that have potent activity across a wide range of resistant Gram-negative pathogens, including species which are part of the ESKAPE pathogens. Amurins have also shown the ability to clear biofilms and act synergistically with a range of standard of care antibiotic agents. Another modality in ContraFect Corporation's pipeline is Lysins as direct lytic agents (DLAs). They can directly bind and cleave the bacterial cell wall and essentially kill on contact. This novel mechanism of action is fundamentally different from antibiotics, which require cell division or metabolism in order to kill bacteria or stop their growth. In addition to their mechanism of action, lysins have a number of key features that differentiate them from antibiotics.
Using bioinformatics and a number of metagenomic-based techniques, the company's internal lysin discovery platform locates and clones bacteriophage lysins from bacterial, viral, and environmental sources. The foundation of the field of metagenomics is the mass extraction of DNA/RNA from environmental samples (such as soil, water, etc.) without the separation of specific microbial sources first. This is important to know because fewer than 1% of microbes can be cultured in a laboratory setting. The metagenomic DNA can then be examined using sequence-based techniques or by specialized functional screens after being extracted. The core of ContraFect's lysin discovery work consists of these functional screens for bacteriophage lysin activity. ContraFect's scientists use a variety of techniques to further refine and "engineer" changes to the lysins after they have been cloned in order to introduce particular properties that we believe may be advantageous for potential therapeutic use.
***
As a concluding note, there are signs of positive changes in the notoriously difficult area of research, including Pasteur Act, emergence of various financial vehicles to fund research, such as AMR Fund, and other initiatives by public, private and government organizations in the US, EU and throughout the world. Also, novel modeling techniques, including machine learning, are increasingly being used to discover novel antibiotics and several successes are reported. Finally, the recent breakthrough in bacterial cultivation with the iChip and teixobactin development gave a new hope for revisiting once successful approaches for antibiotics discovery from natural soils. However, the situation is overall alarming and the rate of bringing novel antibiotics to clinical use, especially for fighting Gram-negative bacteria, must be increased.
In addition to the above, governments around the world must act to reinforce strict regulations regarding antibiotics overuse in medical facilities as well as uncontrolled application in agriculture as this is crucial for decreasing the rate of bacterial mutations and associated resistance development risks.
(This article was first published in 2016, reworked and updated in February 2023)