Novotech Report: Over 800 Global IPF Trials Since 2020, Led by Asia-Pacific
Novotech CRO has released a global market analysis focused on the clinical trial landscape for Idiopathic Pulmonary Fibrosis (IPF), identifying strategic trends for drug developers targeting this chronic and progressive respiratory disease.
The report reviews more than 800 industry-sponsored IPF trials initiated since 2020 and provides region-specific breakdowns, innovation trends, and considerations for trial design.
As of 2022, IPF affects an estimated 13–20 per 100,000 people worldwide, with over one million individuals impacted. Incidence rates vary by region: Asia-Pacific reports 0.35–1.30 per 10,000 (highest in South Korea), North America 0.75–0.93 per 10,000, and Europe 0.09–0.49 per 10,000. Prevalence estimates show a similar spread, with regional differences reflecting varying healthcare systems and diagnostic practices.
(Source: Novotech)
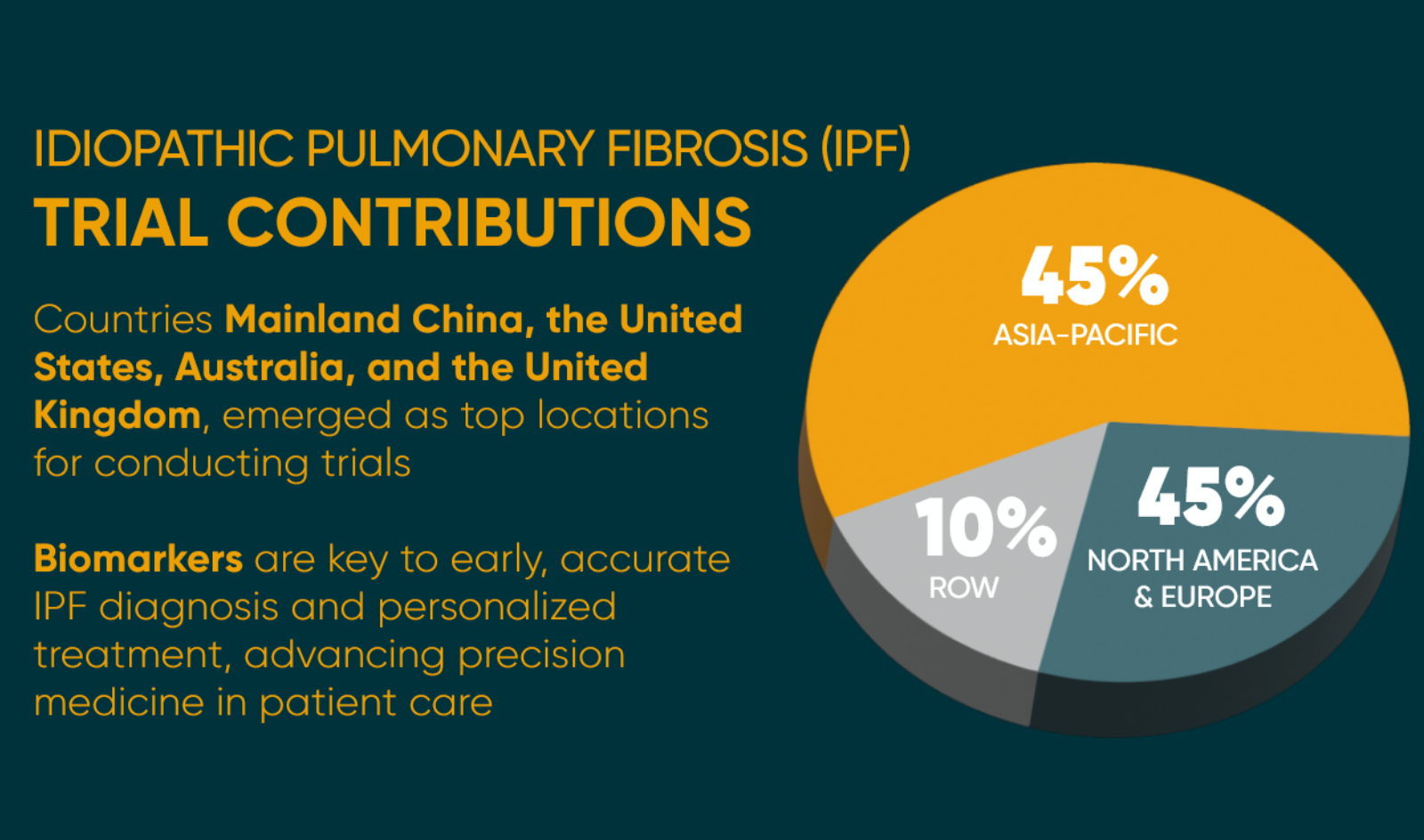
According to the report, the Asia-Pacific region accounts for 44% of IPF trial activity globally, driven largely by high trial volume in China. North America follows with 23%—with the U.S. as the primary contributor—while Europe accounts for 21%, with concentrations in the UK and Germany.
Forced vital capacity (FVC) remains the primary endpoint in IPF trials, with increasing use of secondary measures such as DLCO, patient-reported outcomes, and high-resolution CT imaging. Trials are progressively incorporating biomarker-based stratification—particularly markers like MMP-7, KL-6, and SP-D—to improve cohort selection and monitor therapeutic response.
The report outlines several emerging therapeutic strategies aimed at improving outcomes for IPF, where existing treatment options remain limited.
Small molecule drugs continue to lead development efforts (Refoxy Pharma, Vicore Pharma, Pliant Therapeutics, Cumberland Pharmaceuticals, Endeavour BioMedicines), with new candidates designed to interrupt biological pathways linked to inflammation and lung tissue scarring. Some programs also involve AI-designed compounds, such as those from Insilico Medicine.
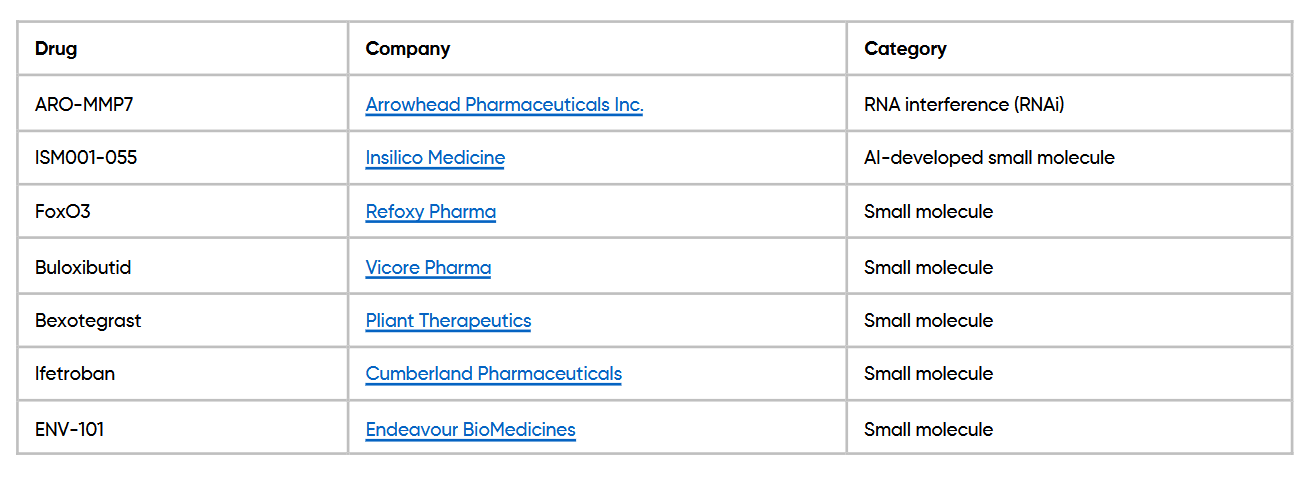
Emerging Therapies and Key Players in IPF Treatment. (Source: Novotec)
New approaches under investigation include RNA interference (RNAi) therapies that aim to block the production of specific proteins involved in fibrosis, as well as inhaled prostacyclin-based treatments intended to reduce tissue damage and improve lung function (Arrowhead Pharmaceuticals Inc.). Additional areas of focus include:
- Monoclonal antibodies that block signaling molecules driving disease progression
- Cell therapies, such as stem cell-based approaches being tested for their anti-inflammatory and regenerative potential
- Pathway inhibitors, including drugs targeting the Hedgehog signaling pathway, which is associated with scar-forming cell buildup
The funding landscape for IPF programs remains active, with strong early-stage investment from venture capital firms and continued public-sector support, notably from the EU Horizon 2020 program and the U.S. Department of Defense.
For a full breakdown—including epidemiology, current standard of care, trial activity by region, evolving trial designs, biomarkers, therapeutic innovations, funding trends, and a SWOT analysis—read the complete report here.
Topics: Contract Research