AI-Driven Robotics Lab Identifies TNIK Inhibition as a Novel Way to Curb Inflammatory Aging
A recent study from researchers affiliated with Insilico Medicine has identified Traf2- and Nck-interacting kinase (TNIK) inhibition as a potential method for reducing the effects of cellular aging. The research implies there's potential for INS018_055, a TNIK inhibitor, to limit the buildup of aging cells and associated inflammation, which are linked to age-related diseases.
The researchers focused on cellular senescence—a process where cells stop dividing and release inflammatory signals that contribute to age-related diseases. Using an AI-driven robotics platform, the team tested INS018_055 and found that it reduced markers of cellular aging in multiple experimental models.
The compound was found to suppress pathways linked to inflammation and tissue damage, suggesting potential benefits for conditions like idiopathic pulmonary fibrosis (IPF), a progressive lung disease associated with aging. Rather than eliminating aging cells entirely, INS018_055 appeared to modify their behavior without negatively affecting cell survival, which could offer a safer approach compared to some existing treatments.
How Robotics Enhanced the Study
The research made extensive use of a fully automated robotics laboratory, which played a key role in managing and accelerating the experimental process.
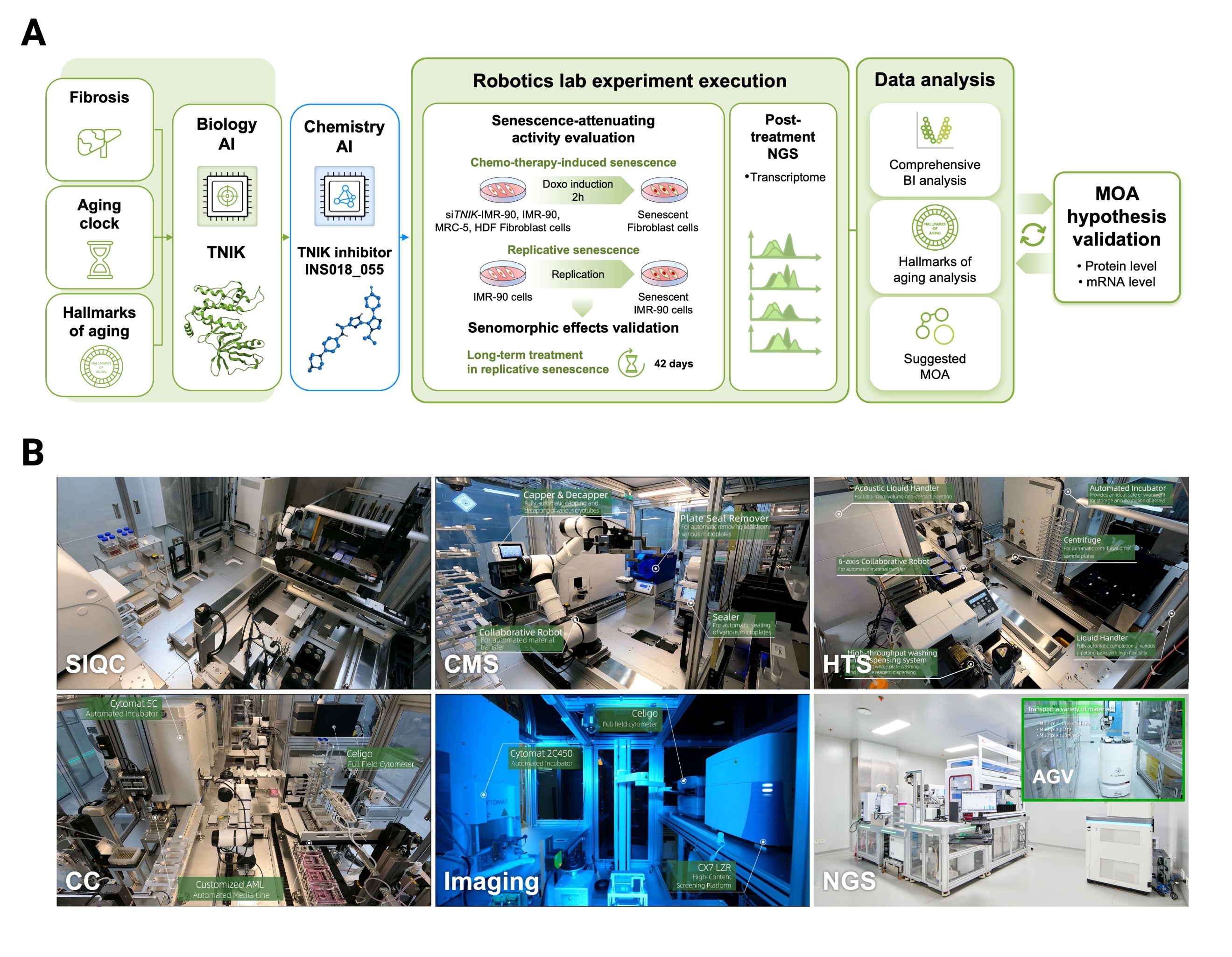
"AI-powered aging research platform and robotics lab-powered workflow. (A) Simplified schematic of project workflow for this study. (B) Layouts of six functional modules of the robotics lab: BI, bioinformatics; NGS, next-generation sequencing; MOA, mode of action; SIQC, sample intake, and quality control; CC, cell culture; CMS, compound management system; HTS, high-throughput screening; NGS, next-generation sequencing. AGV, autonomous guided vehicle" — Study
-
Automated Testing and Data Collection
The robotics system enabled high-throughput screening and analysis across different experimental models. By automating phenotypic and multi-omic analyses, researchers could efficiently assess how TNIK inhibition affected cellular aging and inflammatory responses. -
Integrated Experimental Workflow
The laboratory setup combined multiple automated modules, including sample preparation, compound management, cell culture, high-content imaging, and next-generation sequencing. This allowed for seamless execution of experiments, minimizing human error and increasing reproducibility. -
Efficient Screening for Senotherapeutic Potential
An automated imaging system was used to screen for compounds that reduced markers of senescence. The system could identify senomorphic effects—those that suppress inflammatory signals from aging cells—without relying on manual input, allowing for unbiased, large-scale analysis. -
Accelerated Drug Discovery Process
The robotics platform helped streamline the entire discovery pipeline, from initial target identification to experimental validation. This allowed the team to rapidly test and analyze the effects of INS018_055 across various models of cellular aging.
Potential Applications for Age-Related Diseases
The findings suggest that TNIK inhibition could be relevant for treating diseases where aging cells play a role in disease progression. While the study focused on lung fibrosis, the researchers note that TNIK is also active in other tissues, including the heart, brain, and skeletal muscles, which could expand the relevance of this approach.
INS018_055, already recognized for its anti-fibrotic potential in preclinical models, is currently being evaluated in clinical trials for IPF. Its ability to target aging-related pathways without harming non-senescent cells may provide an alternative to treatments that rely on the elimination of aging cells.
Topics: Novel Therapeutics