Insilico Medicine Reports Benchmarks for its AI-Designed Therapeutics
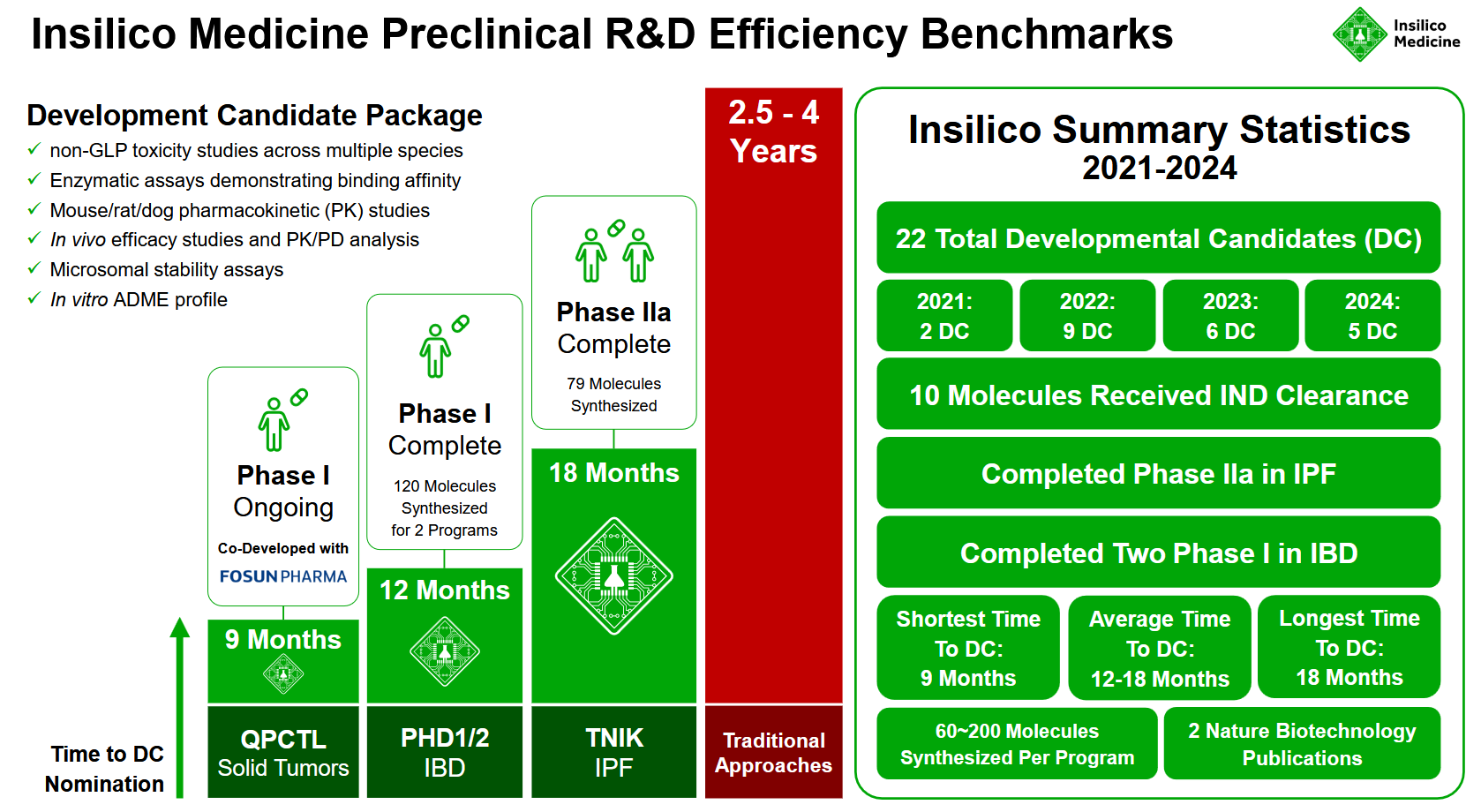
Insilico Medicine has released benchmarks detailing the timelines and development metrics for 22 preclinical candidate (PCC) nominations achieved between 2021 and 2024. These self-reported metrics offer a view of the company's AI-driven drug discovery (AIDD) workflow—demonstrating an average 13-month timeline to PCC nomination, a reduction from the traditional 2.5- to 4-year process, and validating the efficiency of its approach.
By the end of 2024, Insilico Medicine has nominated 22 preclinical candidates, with 10 receiving FDA IND clearance and advancing to human clinical trials. Its latest candidate, ISM5939, an oral ENPP1 inhibitor targeting solid tumors, was developed using its AI-driven Chemistry42 platform and progressed from design to IND clearance in just three months. Insilico also reported a 100% success rate in advancing preclinical candidates to IND-enabling studies, excluding voluntarily discontinued programs.
The company has completed four Phase I studies, and its lead program, ISM001_055 for idiopathic pulmonary fibrosis, recently reported positive Phase IIa results. A Nature Biotechnology paper published earlier in 2024 detailed the entire R&D journey of ISM001_055, from AI-assisted discovery to Phase II clinical trials, providing a case study on Insilico’s developmental candidate package and its AIDD approach.
Source: Insilico Medicine
Benchmarks
- Preclinical candidate nominations (2021–2024): 22
- Average time to PCC: ~13 months
- Molecules synthesized per program (average): ~70
- Longest timeline to PCC: 18 months (79 molecules synthesized)
- Shortest timeline to PCC: 9 months (QPCTL program, co-developed with Fosun Pharma, now in Phase I trials targeting QPCTL proteins to help the immune system engage 'cold' tumors)
The company attributes these timelines to its AI-driven platform, which integrates deep learning models for molecular design, target selection, and preclinical validation.
Candidate nominations at Insilico Medicine include a set of preclinical validation steps before advancing to IND-enabling studies. The developmental / preclinical candidate package consists of:
- Enzymatic assays demonstrating binding affinity.
- In vitro ADME profiling to assess absorption, distribution, metabolism, and excretion.
- Microsomal stability assays to evaluate metabolic stability.
- Pharmacokinetic (PK) studies in mice, rats, and dogs.
- Cellular functional assays and PD marker validation demonstrating target engagement.
- In vivo efficacy studies and PK/PD/efficacy analysis to confirm target engagement and establish efficacious dose ranges.
- 28-day non-GLP toxicity studies in two species.
Source: Insilico Medicine
Case Studies
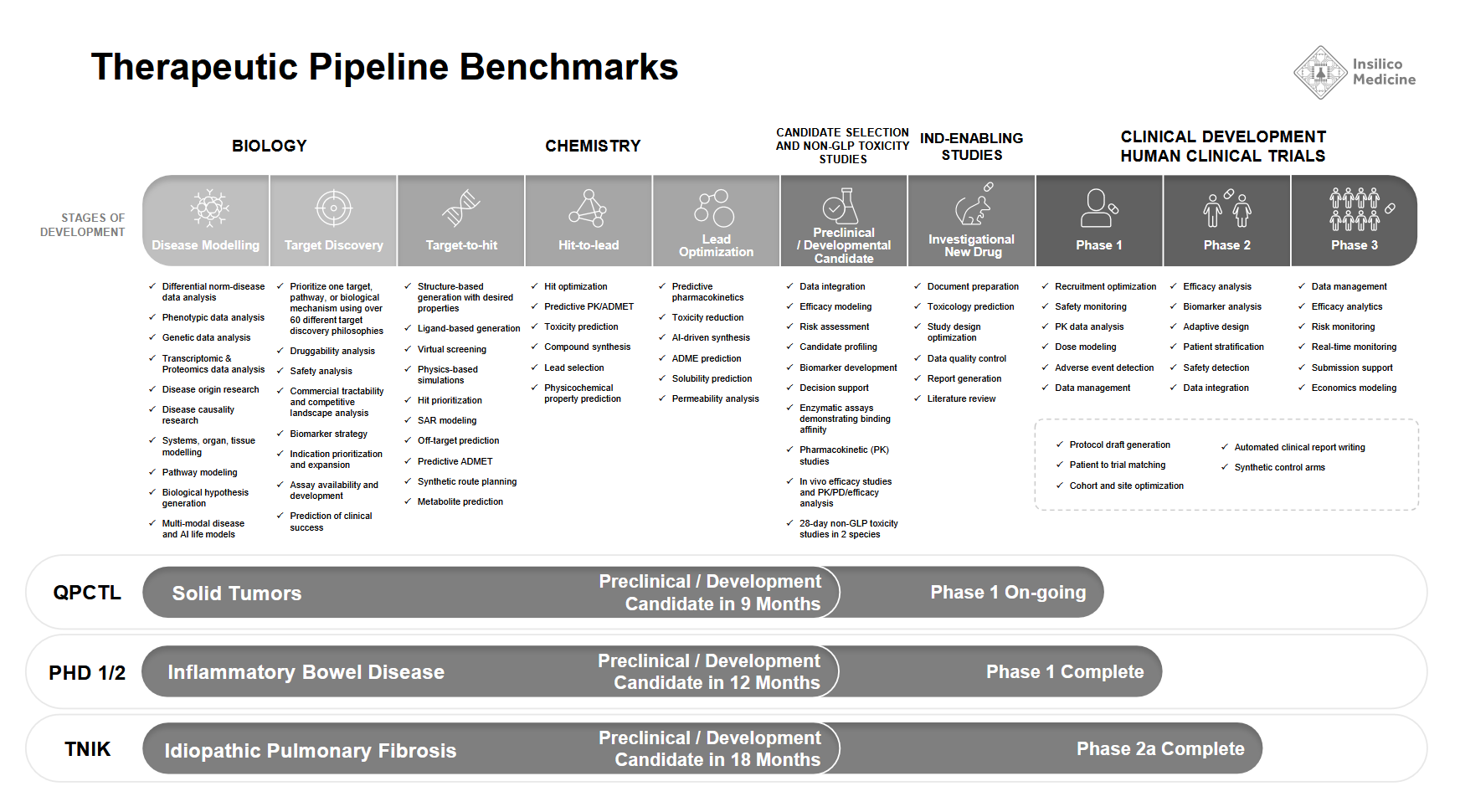
- ISM001_055 (Idiopathic Pulmonary Fibrosis): Nominated in 18 months, this TNIK inhibitor progressed from AI-assisted discovery to Phase II trials. The Phase IIa study, a double-blind, placebo-controlled trial with 71 patients across 21 sites in China, demonstrated safety, tolerability, and dose-dependent efficacy. At the highest dose (60 mg QD), patients showed an average improvement of 98.4 mL in forced vital capacity (FVC) over 12 weeks, compared to a 62.3 mL decline in the placebo group. A Nature Biotechnology article in March 2024 detailed the AI-driven discovery process, including target selection and molecular design. The program is advancing toward pivotal trials, with a U.S. Phase IIa study actively enrolling.
- ISM5411 (Gut-Restricted PHD Inhibitor): Developed over 12 months with 115 synthesized and screened molecules, this candidate was designed to selectively inhibit PHD1 and PHD2, addressing intestinal barrier repair and inflammation in inflammatory bowel disease (IBD) while avoiding the systemic risks—notably cardiovascular and tumorigenic effects—that hindered previous PHD inhibitors. AI-driven structure-based drug design identified critical pharmacophore points and optimized molecular properties to achieve gut-restricted activity. Animal studies confirmed a 67-fold higher concentration in the colon than in plasma, reducing systemic exposure risks. Preclinical models demonstrated efficacy in TNBS- and oxazolone-induced colitis, outperforming standard treatments by improving epithelial integrity and reducing inflammatory cytokine levels. The candidate advanced to Phase I trials in Australia and China, where pharmacokinetic data supported its localized mechanism of action.
Insilico’s AI-based approach is structured to optimize key steps in preclinical development, from early-stage target identification to molecule synthesis and selection. Thus, the company has outlined a framework for streamlining drug discovery by integrating preclinical validation data and running key processes in parallel.
This release of developmental benchmarks echoes the discussion in Progress, Pitfalls, and Impact of AI-Driven Clinical Trials, an article by Dominika Wilczok and Alex Zhavoronkov published in Clinical Pharmacology & Therapeutics in December 2024. The article highlighted the need for transparent benchmarking in AIDD, noting that while AI has accelerated early-stage development, its impact in later clinical phases remains limited.
By publishing detailed data on development timelines and molecular synthesis rates, Insilico seeks to initiate broader industry efforts toward establishing clearer validation standards for AI-designed therapeutics as they progress toward clinical evaluation, addressing a gap in standardized benchmarking within AI-driven drug discovery.
Topics: AI & Digital