Icelandic Biotech Raises €26.5M for Clinical-Stage Dementia and Skin Disease Treatments
Arctic Therapeutics (ATx), an Icelandic drug discovery and development company, has closed an oversubscribed €26.5 million Series A financing round. The funding will support the advancement of ATx’s pipeline targeting rare and common forms of dementia, inflammatory skin diseases, and other conditions.
The financing will primarily accelerate the development of two key programs:
AT-001: An oral treatment designed to disrupt the aggregation of amyloid proteins in the brain, a process implicated in dementia and Alzheimer’s disease. AT-001 works by breaking down disulfide bonds that stabilize harmful protein clusters, preventing their accumulation and subsequent inflammation in brain tissue. The drug is currently in a pivotal Phase IIb/III trial for Hereditary Cystatin C Amyloid Angiopathy (HCCAA), a rare form of familial dementia. ATx plans to explore its potential in broader dementia indications, including Alzheimer’s.
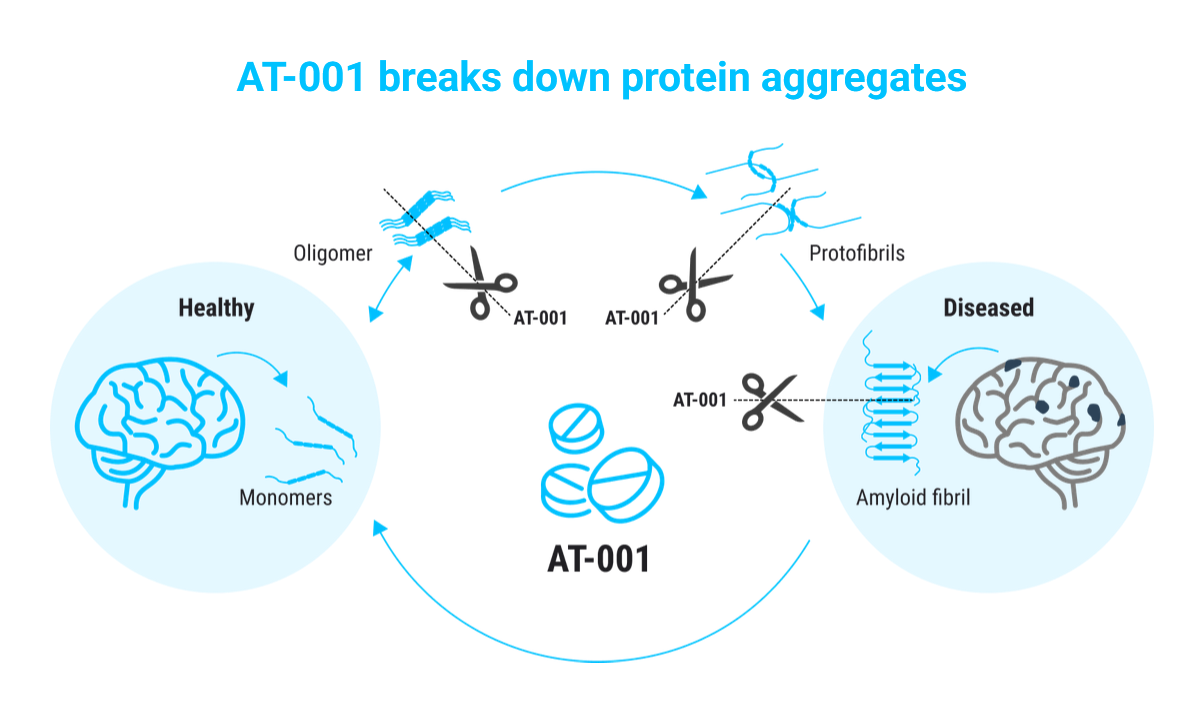
Results from Arctic Therapeutics’ Phase IIa clinical trial, which focused on a specific mutation prevalent in Iceland, have shown promising outcomes. The data indicates that AT-001 has the potential to prevent the rapid buildup of amyloid proteins, reducing the risk of stroke and early-onset dementia. Looking forward, AT-001 aims not only to slow or stop the progression of rare and common forms of dementia but also to potentially delay its onset or even reverse its course. The company has initiated a Phase IIb/III registration study, following approval from the European Medicines Agency (EMA).
AT-004: A topical cream targeting inflammatory skin conditions such as acne vulgaris, atopic dermatitis, and psoriasis. AT-004 operates through a unique mechanism that inhibits acetylcholinesterase, reducing inflammation by modulating the non-neuronal cholinergic system in the skin. The treatment has shown promise in early trials, with plans to initiate Phase IIa studies in Europe later this year.
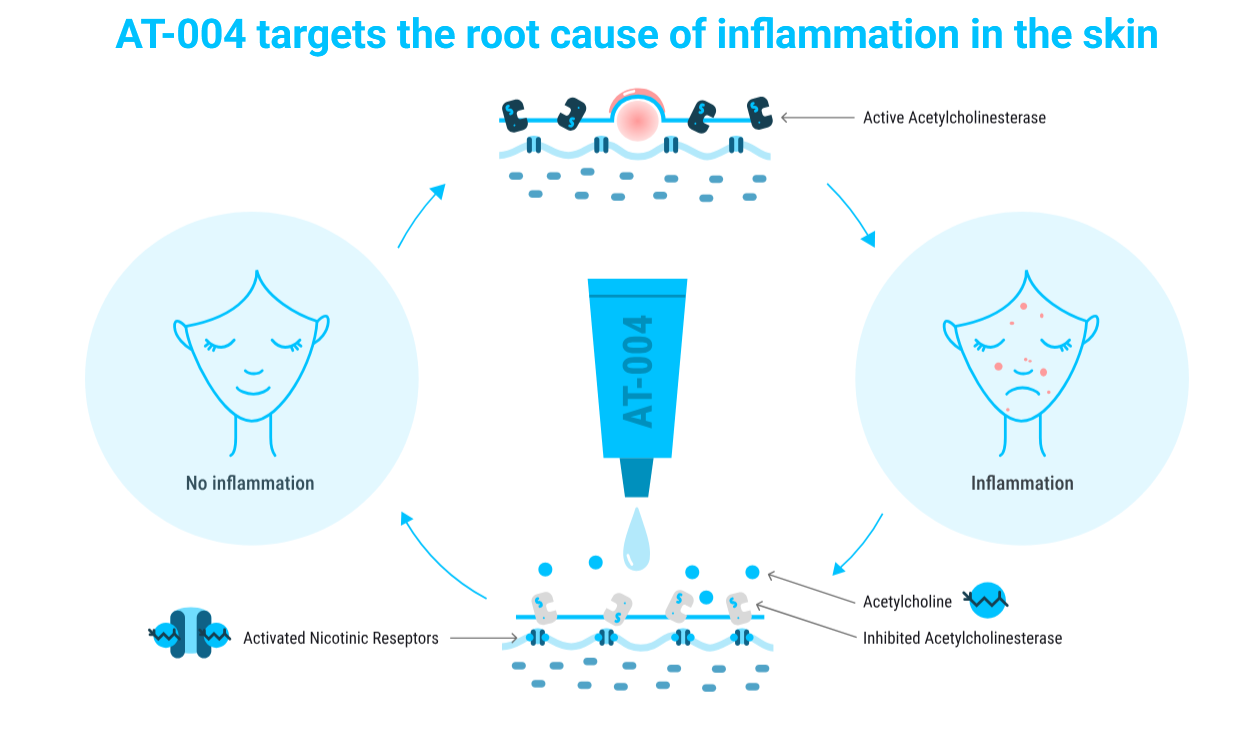
AT-004 works by inhibiting acetylcholinesterase, an enzyme that releases acetylcholine. Acetylcholine then binds to and activates nicotinic receptors, which send signals to halt the production of inflammatory cytokines. This mechanism prevents inflammation from occurring and alleviates existing inflammation, restoring the skin to a healthy state. AT-004 is unique in targeting this pathway and has shown no observed side effects in studies.
In addition to these lead programs, ATx is advancing a broader pipeline of therapies:
-
AT-003: A mitophagy/autophagy modulator designed to treat autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus, as well as Parkinson’s disease. The drug targets dysregulated mitophagy, a process linked to inflammation and neurodegeneration.
-
AT-002: A JAK-STAT inhibitor based on a SOCS1 mimetic peptide, aimed at treating autoimmune uveitis and rare autoinflammatory diseases, including juvenile dermatomyositis. The therapy also has potential applications in managing cytokine storm syndromes, such as those arising from COVID-19 or CAR-T cell therapies.
-
AT-005: An siRNA-based inhalable treatment for severe asthma and rhinovirus type C infections. The drug targets the cellular binding site for rhinovirus, preventing viral entry into the airways and reducing asthma attacks, particularly in patients with a specific genetic mutation in the CDHR3 gene.
ATx’s approach to drug development is rooted in applied genomics and bioinformatics, leveraging Iceland’s unique genetic resources, including a nationwide genealogy database, a biobank licensed by the Icelandic Ministry of Health, and access to encrypted electronic health records. This allows the company to identify genetic drivers of disease and develop targeted therapies with reduced risk and cost compared to traditional methods.
Arctic Therapeutics was recently selected to join the EIC Scaling Club, an initiative supporting Europe’s most promising scaleups. The company’s work aligns with the EIC Fund’s mission to address global healthcare challenges, including the growing burden of dementia, which affects over 10 million people in Europe alone.
Ongoing Clinical Trial for AT-001
AT-001 is currently in a pivotal Phase IIb/III trial for Hereditary Cystatin C Amyloid Angiopathy (HCCAA), a rare form of familial dementia endemic to Iceland. This trial, conducted in collaboration with Landspítali (the National University Hospital of Iceland)
Topics: Novel Therapeutics