Eli Lilly Acquires Scorpion Therapeutics' Oncology Program for $2.5 Billion
Eli Lilly announced the acquisition of Scorpion Therapeutics' mutant-selective PI3Kα inhibitor program for up to $2.5 billion, expanding its oncology pipeline with STX-478, a differentiated PI3Kα inhibitor currently in Phase 1/2 clinical trials.
STX-478 is a once-daily oral therapy designed to selectively target PI3Kα mutations in cancer cells while sparing healthy tissue, aiming to address limitations of existing therapies that often cause significant side effects. This candidate is being evaluated for hormone-positive breast cancer and other solid tumors, with early data suggesting improved tolerability and potential efficacy compared to current treatments.
As part of the transaction, Scorpion will spin out its non-PI3Kα assets into a new company, owned by existing shareholders, with Lilly holding a minority stake. Scorpion’s current team will lead the new entity, which will focus on precision oncology using its proprietary discovery platform. This structure enables Lilly to integrate the PI3Kα program while preserving the momentum of Scorpion’s broader pipeline.
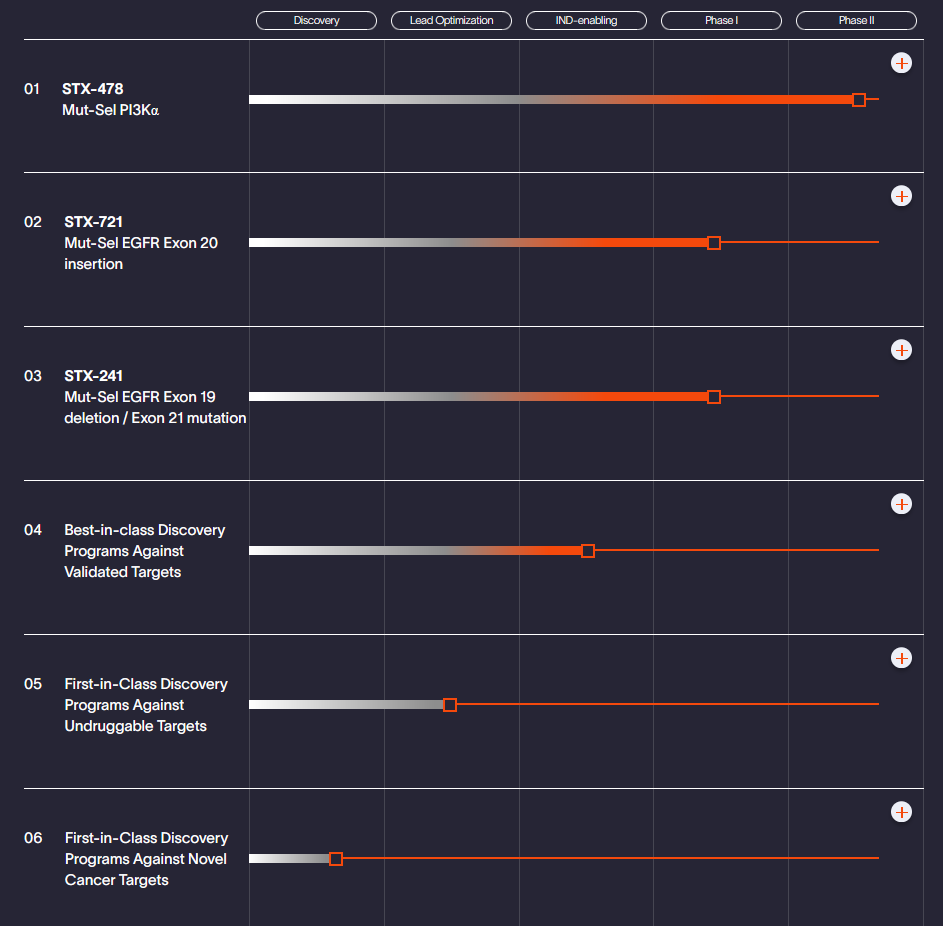
Scorpion Therapeutics' current pipeline (Source: Scorpion Therapeutics)
Scorpion Therapeutics’ pipeline focuses on precision oncology, targeting well-validated and previously undruggable cancer drivers with selective, small-molecule therapies. Besides its leading STX-478 program, the pipeline also includes candidates targeting EGFR mutations, such as exon 20 insertions and C797S resistance mutations in non-small cell lung cancer, as well as discovery-stage programs targeting novel and undruggable cancer mechanisms.
STX-478’s selectivity for mutant PI3Kα could allow deeper pathway inhibition, improved tolerability, and use in combination with other therapies, particularly in earlier-stage hormone-positive breast cancer where unmet needs remain significant. It enters a competitive landscape alongside existing treatments like Novartis’ Piqray and Roche’s Itovebi, offering a potentially safer and more targeted approach. Early clinical data demonstrate promise in overcoming challenges associated with wild-type PI3Kα inhibition, including metabolic side effects and poor CNS penetration, which limit the use of current therapies.
The acquisition positions Lilly to reengage with PI3Kα-focused therapies following the discontinuation of its earlier candidate, LOXO-783, while accelerating its efforts to address cancers driven by these mutations. Lilly’s global resources will now drive further development of STX-478, with the potential to broaden treatment options for patients with PI3Kα-mutated cancers.
Cover photo: Scorpius contellation by allexxandarx - stock.adobe.com
Topics: Startups & Deals