Sana Reports Promising Results for Type 1 Diabetes Islet Cell Transplants Without Immunosuppression
Sana Biotechnology announced promising early results from a first-in-human study of its hypoimmune (HIP) technology for type 1 diabetes. Conducted in collaboration with Uppsala University Hospital, the study evaluated the transplantation of HIP-engineered primary pancreatic islet cells without the use of immunosuppressive drugs. The data suggest that these cells can evade immune rejection, function effectively, and persist in patients.
Type 1 diabetes is an autoimmune disease in which the body’s immune system attacks and destroys insulin-producing beta cells in the pancreas, leading to an inability to regulate blood glucose levels. Islet cell transplantation aims to restore this function by introducing healthy, insulin-producing cells. However, the procedure traditionally requires immunosuppressive drugs to prevent the immune system from rejecting the transplanted cells.
Key findings of the current study include the detection of circulating C-peptide, a marker of insulin production, following transplantation of UP421, a donor-derived allogeneic primary islet cell therapy. Increased C-peptide levels were observed during mixed meal tolerance tests (MMTT), indicating insulin secretion in response to food intake. MRI scans confirmed graft survival 28 days post-transplantation, and no safety issues were reported.
Per-Ola Carlsson, MD, Study Principal Investigator:
“These initial exciting results build upon the extensive preclinical and translational studies of Dr. Sonja Schrepfer and the team at Sana. The clinical data are highly promising for patients and provide the first evidence in humans for overcoming allogeneic and autoimmune rejection with pancreatic islet cell transplantation in type 1 diabetes with no immunosuppression.”
The HIP platform is designed to enable transplanted cells to avoid immune detection, removing the need for immunosuppression. The current study involved intramuscular transplantation of a low dose of islet cells, demonstrating proof-of-concept for this approach. While not yet aimed at glycemic control or insulin independence, the data represent a step toward developing scalable, curative treatments for type 1 diabetes.
Steve Harr, Sana’s President and CEO:
“We achieved our goals for the study, identifying no safety issues as well as demonstrating survival, function, and evasion of immune detection of HIP-modified primary pancreatic islet cells transplanted intramuscularly with no immunosuppression. Safe cell transplantation without immunosuppression has the potential to transform the treatment of type 1 diabetes and a number of other diseases.”
The findings may inform the development of SC451, Sana’s stem cell-derived pancreatic islet cell program. The company plans to continue follow-up on the current study and share the data at scientific forums.
Sana Biotechnology focuses on advancing cell engineering and gene therapy technologies to create transformative therapies for diseases such as type 1 diabetes, B-cell-mediated autoimmune disorders, inherited genetic conditions, and acquired diseases like certain cancers. The company operates on two key fronts: ex vivo and in vivo cell engineering.
In the ex vivo approach, Sana is developing methods to repair or replace damaged or missing cells, such as pancreatic islet cells for diabetes or allogeneic CAR T cells for autoimmune and oncology applications. This involves:
- Differentiating stem cells into specific, clinically needed cell types.
- Addressing key challenges like scalable cell production, functional integration, and immune rejection.
- Overcoming immune rejection from "non-self" cells, considered the most significant barrier to making cellular therapies broadly accessible.
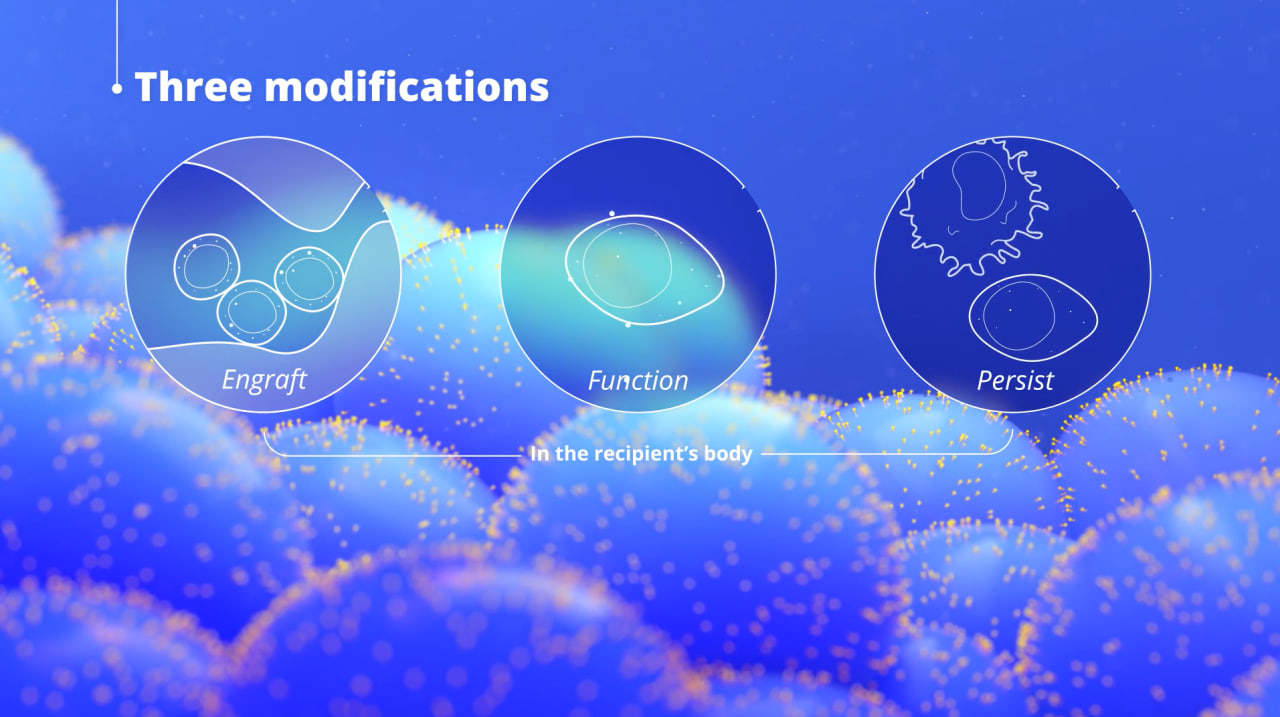
Reportedly, with "only three modifications, cells become hypoimmune, and can engraft, function, and persist in the recipient's body." (i.e. by modifying cells to overexpress CD47, a protein that signals macrophages—the immune cells responsible for engulfing and destroying aging or diseased cells—not to attack. By increasing CD47 expression, transplanted cells can evade immune detection and rejection, mimicking the natural mechanism used by healthy cells to protect themselves from immune-mediated destruction. (Source: Sana Biotechnology)
In the in vivo approach, Sana aims to deliver genetic modifications directly into patients’ cells via modified fusosomes to treat conditions such as single-gene disorders or acquired diseases requiring precise genetic alterations. This involves three core components:
- Delivery: Developing the capability to deliver therapeutic payloads to targeted cells in a specific, predictable, and repeatable manner.
- Gene Modification: Advancing techniques such as gene editing, base editing, gene insertion, and gene expression control to address a broad spectrum of diseases.
- Execution: Ensuring scalable and consistent product manufacturing, designing strategic clinical trials, and addressing the technical requirements for delivering therapies to patients effectively.
Sana’s research includes the use of engineered fusogens, which are cell-targeting proteins found in viruses and mammalian cells, to enhance the precision of gene delivery. By re-engineering the fusogen G protein, Sana can direct therapeutic payloads to specific cell types by targeting receptors on their surface. This targeted approach reduces off-target effects that are common with conventional delivery technologies, such as AAVs and LNPs, and enables applications like modifying hematopoietic stem cells for genetic disorders or delivering therapeutic genes to alter cellular behavior in other diseases.
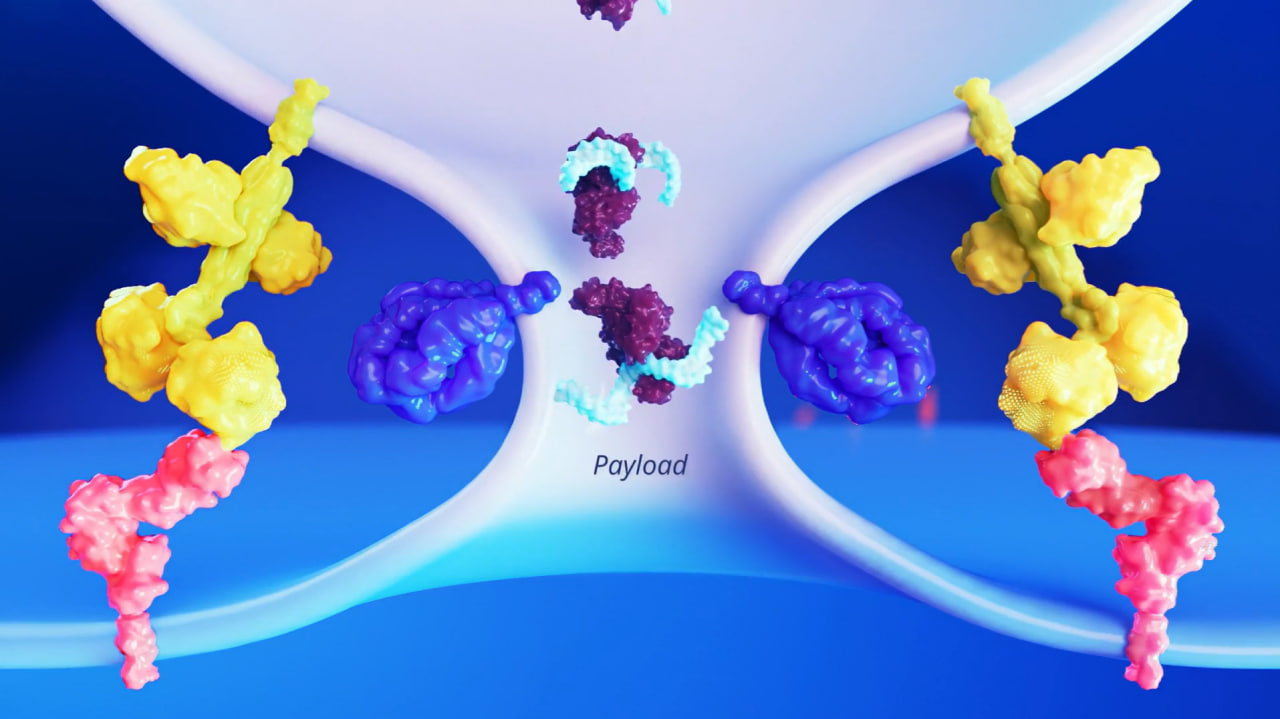
Illustration of Sana's fusogen-based gene delivery technology: Engineered fusogens precisely target specific cell surface receptors, enabling the direct delivery of therapeutic payloads into the cytosol while minimizing off-target effects. (Source: Sana Biotechnology)
Sana's current pipeline highlights a diverse range of advanced programs that leverage its hypoimmune and cell engineering technologies. These include SC291, an allogeneic CAR T therapy for B-cell-mediated autoimmune diseases such as lupus nephritis and ANCA-associated vasculitis, currently in a Phase 1 trial (GLEAM). Another key program, SC262, targets CD22+ cancers like non-Hodgkin lymphoma and acute lymphoblastic leukemia and is being evaluated in the Phase 1 VIVID study. For type 1 diabetes, Sana is advancing SC451 (preclinical), a stem cell-derived pancreatic islet cell therapy, alongside UP421, a first-in-human safety study of primary islet cells engineered with hypoimmune technology.
Cover image: Sana Biotechnology
Topics: Clinical Trials