Eli Lilly Partners with UK’s Alchemab to Develop New ALS Antibodies
Eli Lilly has partnered with UK-based biotech Alchemab Therapeutics to develop therapeutic antibodies targeting amyotrophic lateral sclerosis (ALS), a neurodegenerative disease with limited treatment options. The agreement comes during a difficult period for ALS drug development, as recent clinical trials have seen high-profile failures.
Under the deal, Lilly and Alchemab will work on up to five antibody candidates, leveraging Alchemab's antibody discovery platform. The terms include an undisclosed upfront payment to Alchemab, with potential milestone payments for discovery, development, and commercialization, as well as royalties on any successful products.
Alchemab’s platform focuses on identifying naturally occurring protective antibodies, leveraging "Nature’s most effective search engine"—adaptive immunity—to uncover immune responses that confer protection. By analyzing the antibody repertoires of individuals who exhibit resilience to specific diseases, such as slow-progressing neurodegenerative disorders or long-term cancer survival, the platform reveals insights into protective immune mechanisms.
This approach begins with identifying resilient groups, such as long-term cancer survivors or patients with slow-progressing neurodegenerative disorders. B cells from these individuals are sequenced, generating billions of antibody sequences that are fed into a computational drug discovery engine. Machine learning models and data-mining techniques are employed to pinpoint functionally related protective antibodies convergent across resilient populations. The binding targets of these antibodies are then studied to understand their protective properties, leading to the development and optimization of therapeutic candidates designed to replicate these effects in patients suffering from hard-to-treat diseases.
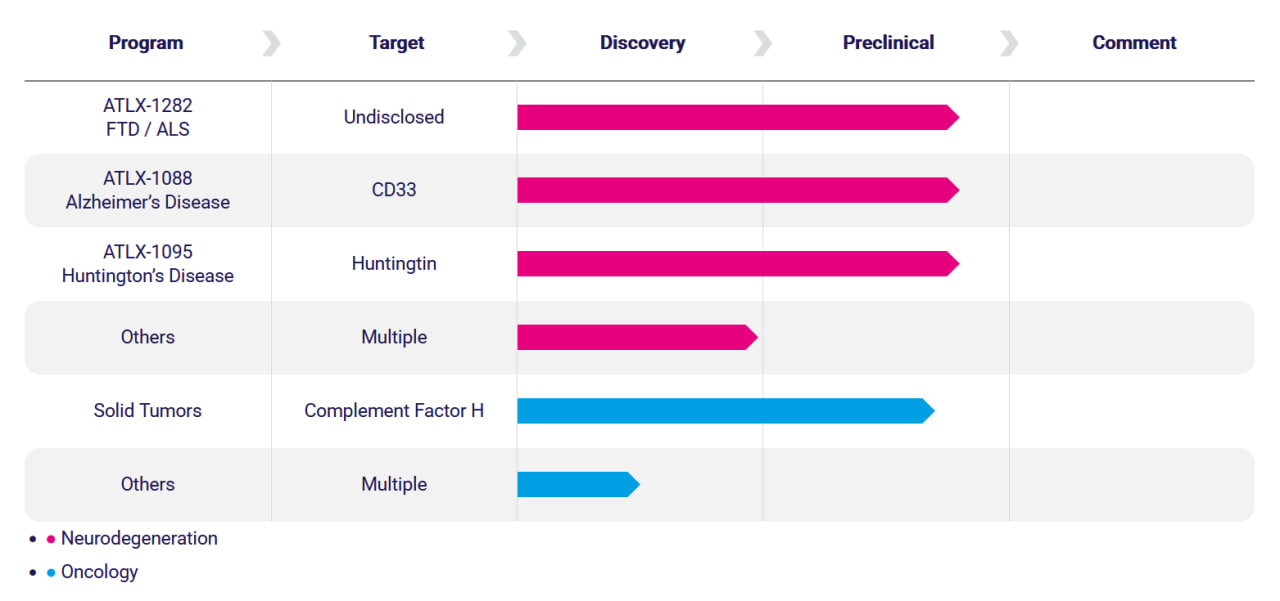
Alchemab's pipeline includes neurodegeneration and oncology programs targeting ALS, Alzheimer's, Huntington's, and solid tumors
Jane Osbourn, Ph.D., CEO of Alchemab:
“We have shown that our novel, differentiated antibody discovery platform can lead to insights into how an individual's immune response can generate potent, selective, and unique antibodies with therapeutic potential. The platform not only identifies potential therapeutic targets but also generates antibody candidates for development in a single process.”
ALS, also known as Lou Gehrig’s disease, remains an area of significant unmet need, with most patients succumbing to the disease within two to four years of onset. Recent investigational therapies from AbbVie-Calico and Denali failed to demonstrate efficacy in slowing disease progression in clinical trials. Lilly’s direction in ALS drug development follows its $45 million deal with QurAlis in late 2024 to develop preclinical candidates for ALS and dementia.
Cover image: iStock
Topics: Novel Therapeutics