Sponsored by SpiroChem
Chemistry CROs at a Crossroads: How SpiroChem Is Adapting to Market Shifts in 2025 and Beyond
As we approach 2025, the role of chemistry Contract Research Organizations (CROs) is undergoing profound transformation. Chemistry is no longer considered a commodity but an essential component in the discovery of new medicines. Biotech companies are no longer satisfied with "more chemists" or "bigger libraries"; they now demand partners with specialized expertise to tackle the increasing complexity of chemical research, which has become the new norm for small molecules, and even more so for emerging modalities such as bRo5 molecules, PROTACs, antibody-drug conjugates (ADCs), and radiopharmaceuticals. These new modalities call for advanced capabilities in macrocycles, linker design, and bifunctional agents—demanding a redefinition of CRO capabilities.
Simultaneously, the way biotech firms engage with CROs is evolving. Traditional Full-Time Equivalent (FTE) and Fee-for-Service (FFS) models are being replaced by flexible, results-driven collaborations. Geopolitical shifts further complicate the global CRO landscape, as reliance on Chinese providers declines and Indian CROs absorb much of the shifting volume. This dynamic creates opportunities for specialized players to assume a critical role within a "tripartite CRO strategy," balancing cost-efficiency, expertise, and strategic resilience.
In this article, I explore these trends and demonstrate how premium CROs do position themselves as indispensable partners in an industry defined by complexity, opportunity, and transformation.
The Rise of bRo5 and Hybrid Modalities
Let’s start with evolving modality trends. Traditional drug discovery thrived with Lipinski’s "Rule of 5" small molecules—simple, low-molecular-weight structures that were relatively easy to synthesize. However, the stringent "dogma" imposed intrinsic limitations on the structural complexity of molecules, but the days of "low-hanging fruit" are over. Challenging drug targets, such as protein-protein interactions (PPIs), E3 ligases, and RNA-binding pockets, require larger, more complex, and more polar molecules that break many, if not all, of these rules. The rise of "beyond Rule of 5" (bRo5) modalities—including PROTACs, macrocycles, cyclic peptides, radioligands, and ADCs—is transforming the outsourcing landscape.
Take macrocycles, for example. With their unique 3D structures, macrocycles excel in modulating "undruggable" targets like PPIs, making them crucial for oncology and antiviral therapeutics. Recent successes, such as the FDA approval of Zilucoplan (2023) for generalized myasthenia gravis and Merck’s MK-0616—a PCSK9 inhibitor—highlight their therapeutic potential. This latter example is a masterpiece from a structural point of view and shows that “when there is a will, there is a way,” even for complex molecules. However, designing and synthesizing macrocycles efficiently remains a significant challenge.
At SpiroChem, we have invested heavily in this area. Our acquisition of Cyclenium Pharma brought us the SpiroQUEST™ Library, a proprietary collection of 8,000+ "next-generation" macrocyclic scaffolds. Additionally, our SpiroBrain™ knowledge model and computational design tools enable efficient exploration of chemical space, reducing synthesis cycles and accelerating timelines. Leveraging our high-throughput parallel synthesis platform, we deliver macrocycles at scale and tailored to client-specific needs.
Next, there is a rise of proximity inducing modalities, like PROTACs (Proteolysis Targeting Chimeras) and such modalities are changing the demands placed on chemical CROs. Recent milestones highlighting the promise of proximity inducing compounds include the advancement of vepdegestrant (ARV-471), a PROTAC targeting the estrogen receptor, into Phase 3 clinical trial for metastatic breast cancer, and the development of luxdegalutamide (ARV-766), an oral androgen receptor degrader, showing promise in treating metastatic castration-resistant prostate cancer.
These molecules are no longer just “small” or “large” — they’re hybrids of multiple functional domains. A PROTAC, for example, has a “warhead,” a linker, and an E3 ligase binder — and each of these elements must be precisely tuned.
Linker design alone could “make it or break it” when it comes to complex drug modalities like PROTACs, ADCs, bioconjugates, oligonucleotides, or radioligands.
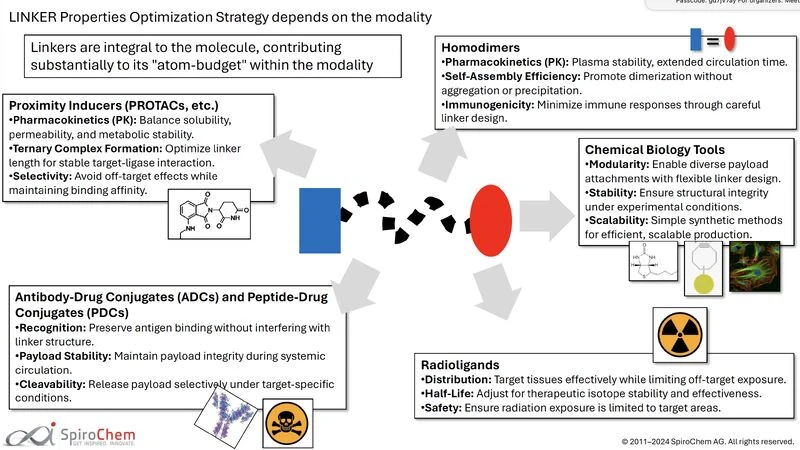
At SpiroChem, we specialize in designing and producing custom linker libraries. But it’s not just about having a library — it’s about having a partner that can create novel linker designs on demand.
Our Hercules+™ high-throughput synthesis platform allows us to generate focused libraries of analogues for linker optimization. This approach is tailored to biotech companies that don’t want to get bogged down in linker design but still want precise control over their PROTAC development program.
Some drug discovery areas are increasingly dominated by biologics, including well-known success stories like adalimumab (Humira) for rheumatoid arthritis and pembrolizumab (Keytruda) for cancer immunotherapy, but biologics have their unique limitations as well. In this article, I won’t be focusing on biologics specifically, but there is clearly a demand for chemical input there, for very promising hybrid modalities like antibody-drug conjugates (ADCs).
As of today, 14 ADCs are FDA-approved and over 300 are currently in clinical trials, with potentially above a thousand projects in development globally. Recent milestones in ADCs include the FDA's approval of mirvetuximab soravtansine-gynx (Elahere) in November 2022 for platinum-resistant ovarian cancer, and the anticipated approvals of datopotamab deruxtecan (Dato-DXd) and patritumab deruxtecan (HER3-DXd) in 2024–2025, targeting various solid tumors.
In the case of ADCs, one of the bottlenecks is in the conjugation chemistry. Linker-to-antibody attachment requires sophisticated control of reactivity, specificity, and stability. The right linker is critical to success, and SpiroChem’s track record in covalent attachment chemistry positions us as a key chemical partner for ADC developers.
Finally, radiopharmaceuticals are on the rise too, and they present yet another layer of complexity for a chemical CRO. These molecules combine small-molecule targeting agents with radioactive isotopes for cancer diagnostics and therapy.
Recent advancements in radiopharmaceuticals include the FDA's approval of Flyrcado in September 2024, a radioactive diagnostic drug for detecting coronary artery disease, and Eli Lilly's $1.4 billion acquisition of POINT Biopharma in October 2023, enhancing their portfolio with late-stage radiopharmaceutical therapies.
From Bristol Myers Squibb to Eli Lilly, major players are making bold bets on radiopharmaceuticals, inspired, for example, by Novartis’ Lutathera—a macrocyclic peptide radioligand that has set a new standard in precision oncology.
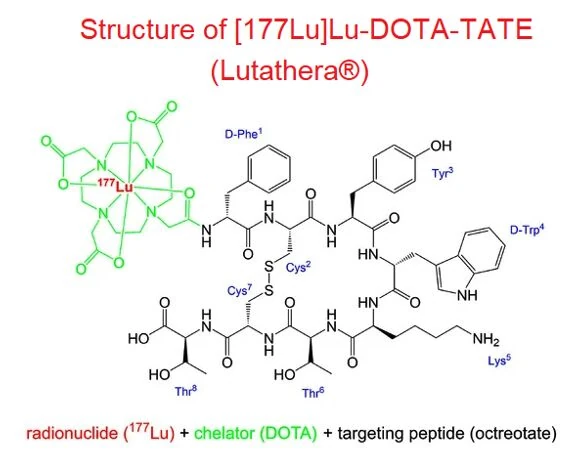
But developing a radiopharmaceutical isn’t just about attaching a radioactive tag — the stability, solubility, and pharmacokinetics of the molecule are just as critical. SpiroChem's expertise in synthesizing bifunctional chelating agents—which securely bind radiometals to targeting vectors—is crucial for the stability and efficacy of metal-based radiopharmaceuticals. Our team’s proficiency in late-stage functionalization enables precise attachment of these chelators to biomolecules, ensuring optimal pharmacokinetics and biodistribution in diagnostic and therapeutic applications.
The Rise of AI and the Power of Data
Artificial intelligence (AI) is revolutionizing life sciences, reshaping how data is utilized in drug discovery and beyond. This year’s Nobel Prize in Chemistry recognized AI’s transformative impact, underscoring its pivotal role in accelerating research and enabling breakthroughs. At SpiroChem, we are embracing this shift through innovative integration of AI and machine learning (ML) into our processes.
Our SpiroBrain™ platform merges our team’s collective human intelligence with cutting-edge AI/ML solutions to accelerate discovery. We are AI-ready and have curated and built a database of millions of reaction data points to feed our SpiroBrain™. A standout example is our collaboration with the University of Geneva, where we developed a generative AI tool trained on proprietary SpiroChem R&D data. This tool powers SpiroSPACE™, a platform that creates ENABLED virtual chemical spaces, allowing virtual screening and opening new frontiers for exploration and innovation in drug discovery. By combining human expertise with advanced AI, we aim to deliver faster, smarter solutions to our partners.
Evolving CRO Business Models
With all the above mentioned trends in chemical modalities, chemistry is only part of the story. The drug discovery partners are increasingly looking for better flexibility of their work with CROs, especially when it comes to startups or medium-sized companies, and highly complex drug design projects at large pharma/biotech.
Traditional CRO relationships were defined by the number of FTEs (Full-Time Equivalents) a client could "rent." The FTE model offers dedicated, continuous support, making it ideal for long-term, iterative projects like lead optimization. Clients benefit from better control and flexibility, but since payments are based on time, not outcomes, it risks inefficiency if project goals aren’t met quickly.
In contrast, another popular approach, the FFS (Fee-for-Service) model ties payments to specific deliverables, transferring risk to the CRO. It works best for short-term, well-defined tasks like custom synthesis or small library production. However, project deprioritization can occur if synthesis is too complex, and costs may increase if multiple iterations are required.
Our approach at SpiroChem is different. When applicable, we offer a results-oriented pricing model that allows our partners to pay for what matters — deliverables, not hours, but maintain the benefits and long-term stability if necessary. This hybrid approach blends Fee-for-Service (FFS) pricing for clear, fast-turnaround tasks (like focused libraries) with FTE contracts for exploratory, undefined projects (like PROTAC discovery).
For high-impact projects where risk is high, we offer ROI-based pricing, where a portion of our fee is tied directly to the successful delivery of key deliverables. It’s a bold approach, but it reflects our confidence in our ability to deliver results, not just effort, and our belief in measuring productivity by outcomes, not hours.
For instance, using our Hercules+™ high-throughput synthesis platform, we routinely deliver focused libraries of tens or hundreds of analogues, for “simple” to “medium” complexity. We use an FFS model at the start, only switching to FTE when molecules require more complex tuning or scaffold editing.
See below two examples that our platform would render “FFS-compatible”: we can make tens or hundreds of analogues per 2-4 week cycle for the PROTACs (linker optimization) or 4-6 week cycle for the macrocycle.
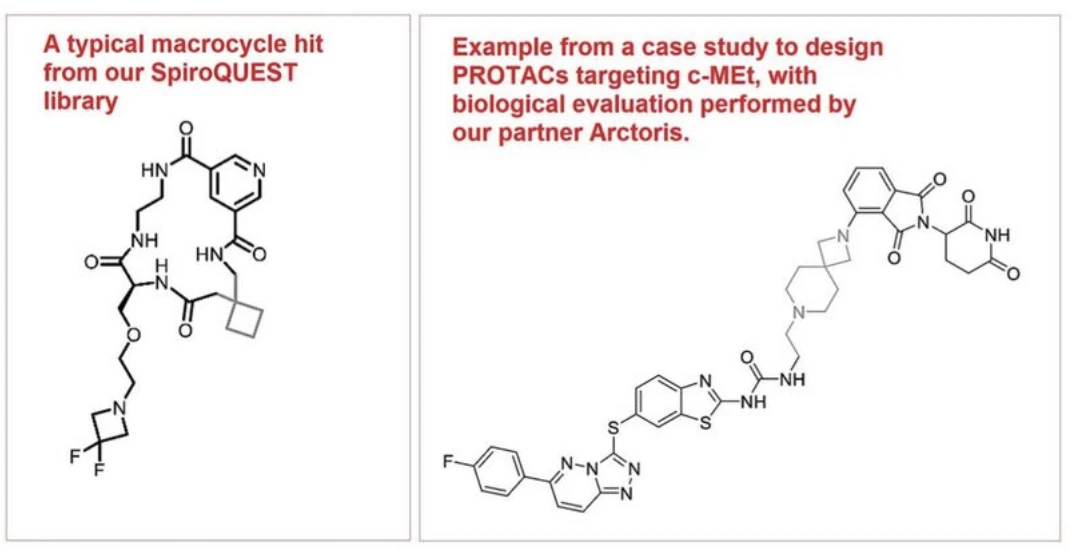
To be able to sustain highly complex but economically efficient endeavors, we also constantly invest in our technology platform -- not only creating internal know-how and processes, like SpiroBrain, Hercules+™ or SpiroQUEST™, but also plugging in cutting-edge capabilities from our partners.
One recent example is our strategic collaboration with Oxford-based Arctoris, a fully automated biology screening CRO that combines robotics-driven lab automation with artificial intelligence (AI)-powered data analytics to generate high-quality, reproducible experimental data for hit identification, lead optimization, and preclinical development.
Their Ulysses® platform gives us access to fully automated, end-to-end biology workflows that can run experiments around the clock. Combine that with our advanced synthetic and medicinal chemistry capabilities, and you have a system and a business offering where molecules are designed, made, tested, and optimized in one integrated process, following a flexible ROI-driven pricing model.
The value of this partnership was further demonstrated by the recently-announced strategic alliance with Orion Pharma (Finland), plugging our joint capabilities into Orion’s established pharma platform and bringing significant acceleration to their well established therapeutic development expertise and clinical infrastructure into the fold. Such model can be reproduced with more Biotech and Pharma clients, offering high impact and a truly end-to-end drug discovery platform—where design, synthesis, biological testing, and candidate optimization happen in a fully integrated, data-driven cycle, accelerating the discovery of bRo5 molecules, PROTACs, and macrocycles for high-impact therapeutic areas like oncology and chronic diseases.
Rethinking Supply Chains Amid Geopolitical Shifts
In recent years, geopolitical shifts have brought about a fundamental change in how biotech and pharma companies engage with CROs. The COVID-19 pandemic, U.S.-China trade tensions, a war in Eastern Europe, and other global disruptions have exposed vulnerabilities in over-reliance on single-region providers. As a result, companies are rethinking their outsourcing strategies — not in terms of supply chain logistics, but in how they distribute critical R&D partnerships to reduce dependency and increase resilience.
One clear outcome of this shift is the gradual reduction of reliance on Chinese providers, driven in part by new regulatory pressures such as the U.S. BIOSECURE Act, which aims to strengthen domestic control over critical biotechnology supply chains. These measures are pushing biotech and pharma companies to diversify their outsourcing strategies, not as a choice but as a necessity. No matter your feelings about these new regulations, the impact is undeniable: the industry is shifting rapidly, and companies that fail to adapt risk being left behind.
As reliance on Chinese providers declines, I expect much of the volume is being absorbed by Indian CROs. But beyond this, we are seeing the emergence of a "tripartite CRO strategy" — a model where biotech companies diversify their R&D partnerships across three tiers: high-volume capacity providers (like Indian CROs), regional specialized CROs with deep scientific expertise, and clients that possess internal know-how and contribute alongside specialized CROs to the intellectual development of projects, benefiting from a reduced need for high-level and in-house experienced staff, which is provided by the specialized CRO. In this model, the specialized CRO acts as a coordinator of activities on behalf of the Client.
At SpiroChem, we see a unique opportunity to position ourselves within this evolving model. With our roots in Switzerland and an expanding footprint in North America (following the Cyclenium deal), we offer biotech and pharma companies an agile, science-first partner. While large CROs focus on capacity, our emphasis is on high-value chemical expertise, particularly in areas like bRo5 molecules, PROTACs, and precision linker design. Our proximity to European and North American biotech hubs provides companies with a strategic alternative to the "volume-over-value" outsourcing model.
This tripartite strategy is still taking shape, but the direction is clear: biotech companies are moving toward a more distributed, multi-tiered approach to outsourcing. For those of us operating in the space, this shift is not about becoming the biggest player but about being the most essential player. In this new reality, we believe there is a growing role for CROs that can combine technical depth with geographic flexibility, and we see ourselves fitting squarely into that role.
Sustainability: A Must-Have Imperative
Last but not least, sustainability has become a central concern for the industry—and for us at SpiroChem. The conversation has shifted from "nice to have" to "must have," driven by stringent ESG (Environmental, Social, Governance) criteria imposed by regulators, investors, and customers alike. Biotech and pharma companies must now align their processes with these standards, and CROs are no exception.
For us, sustainability isn’t just about ticking boxes. It’s about designing smarter synthesis routes that deliver real impact. For example, identifying synthetic shortcuts to reduce the number of reaction steps from seven to three cuts not only time and cost but also energy, water, and solvent use. Scaled across tens of thousands of molecules each year, this results in significant energy savings—and it’s not hypothetical. This kind of efficiency is happening in our labs right now, where green chemistry meets scientific ingenuity.
What holds true for discovery chemistry applies even more strongly to PR&D (Process Research and Development). At SpiroChem, our PR&D team has become a go-to resource for biotech, pharma, and even CDMOs. We specialize in designing and testing new scalable, IP-protected, and cost-efficient synthetic routes, helping our clients meet their sustainability goals without compromising on performance or innovation. These efforts not only enhance environmental outcomes but also align with the broader industry demand for lean, efficient, and scalable production processes.
SpiroChem’s Place in the Evolving Industry
So, where does this leave SpiroChem in the grand scheme of things? I believe we’re at the right size to have a real impact.
With around 100 exceptional chemists and scientists, we’re big enough to scale up and support global pharma projects, but we’re still small enough to stay fast and flexible. Over the last few years, we have demonstrated our ability to grow our team and recruit complementary talents without dilution of quality. We leave no room to error, and our rigorous recruitment process has demonstrated its value.
While some companies are focused on high FTE-count projects, we’re playing a different game. Our value lies in the ability to tackle any chemistry, from simple to complex, and contribute to the design of new medicines. It’s not about competing on cost; it’s about competing on value, uniqueness and speed.
Looking back, it is probably what has differentiated SpiroChem from other players all these years. There are good chemists in other CROs, no doubt. But the concentration of talents that we have been able to assemble at SpiroChem has allowed us to reach a critical mass that can solve virtually any problem. This is our DNA.
Looking ahead, I think the demand for high-value, high-complexity chemistry is only going to increase, especially with novel shifts into the realm of computational modeling, novel target classes, and more ambitious de-novo concepts.
Companies want to reduce their exposure to risk, but they still need access to specialized capabilities. Supply chains are going to stay regionalized—especially for essential medicines and early-stage drug discovery. This is where SpiroChem fits in. With our footprint in Switzerland and North America, we’re able to offer our customers a "closer-to-home" supply chain. We’re not just reducing risks for them—we’re helping them sleep better at night knowing they have a highly sophisticated, stable, transparent, and compliant partner in their corner. This "closer-to-home" supply chain for discovery and early PR&D emphasizes the stability of operations and reliability, a trademark of Swiss-born companies. In a rapidly evolving industry, SpiroChem stands as a beacon of innovation and reliability.
The next five years will be among the most exciting in the history of drug discovery. The new paradigms of technology and collaboration are unlocking possibilities that were once unimaginable. Together, these advancements are enabling the development of better drugs, faster, and ensuring they reach patients in need more efficiently. As an industry, we are on the cusp of transformative change, and SpiroChem is proud to be at the forefront of this journey.
Topics: Contract Research