Gameto Achieves First Live Birth Using Egg Maturation Technology Outside the Body
Gameto, a biotechnology company focused on fertility treatments, announced the world’s first live birth using Fertilo, its ovarian support cell (OSC) technology that matures eggs outside the body.
The delivery took place at Santa Isabel Clinic in Lima, Peru, demonstrating Fertilo’s potential to reduce reliance on traditional in-vitro fertilization (IVF) protocols. Fertilo uses engineered, young ovarian support cells to replicate the natural egg maturation process in a laboratory setting. This approach reduces hormone injections by approximately 80%, shortening IVF or egg freezing cycles from 14 days to just three days.
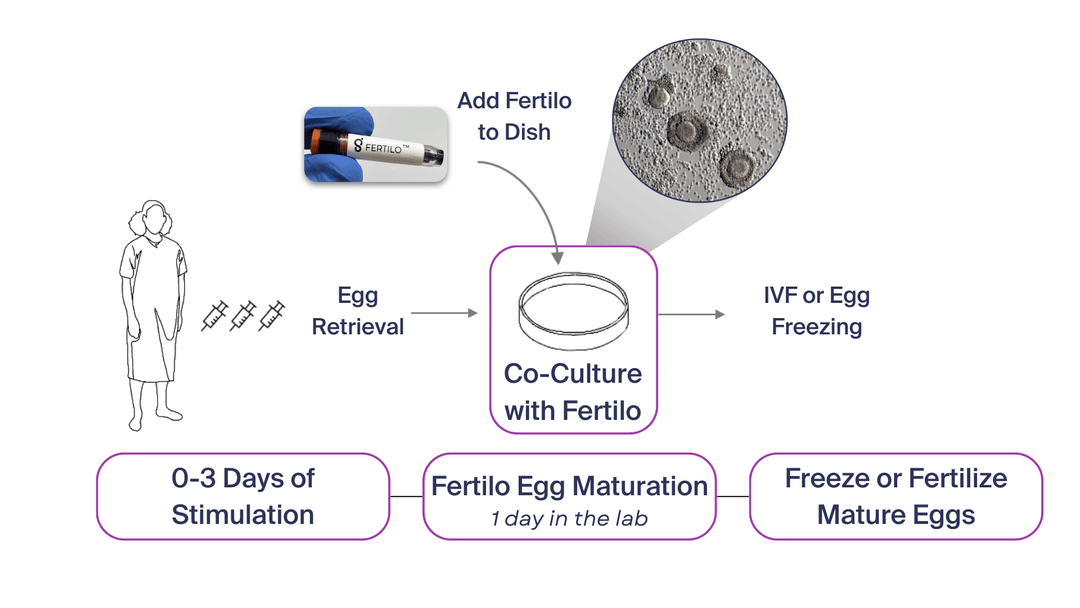
Image: Gameto
Fertilo works by co-culturing immature eggs with ovarian support cells in a dish to facilitate maturation outside the body, mimicking the body’s natural process. This method could replace the need for prolonged hormonal stimulation, potentially reducing risks such as ovarian hyperstimulation syndrome (OHSS) and alleviating side effects associated with high hormone doses.
Dr. Luis Guzmán, Lead at Pranor Labs & Science, who oversaw the Fertilo-enabled IVF cycle, explained:
“The ability to mature eggs outside the body with reduced hormonal intervention offers an alternative for women who cannot tolerate or prefer to avoid traditional IVF protocols. This method may broaden treatment options and improve accessibility for more patients.”
The mother of the first baby born using Fertilo shared her experience:
“The Fertilo method was the preferred option compared to traditional approaches. With fewer injections and a gentler, less invasive egg retrieval process, it gave me hope and reassurance during a deeply personal journey. Fertilo’s approach eased both the physical and emotional challenges, and I’m grateful to the Pranor Clinic and Gameto team for their care.”
Gameto has received regulatory clearance for Fertilo in markets including Australia, Japan, Argentina, Paraguay, Mexico, and Peru. The company recently partnered with IVFAustralia, part of the Virtus Health group, to advance availability of the technology. Phase 3 clinical trials are being prepared in the United States to further assess its efficacy and safety.
Topics: Biotech