Insilico Medicine Nominates its 21st Pre-Clinical Candidate: An Orally Available NLRP3 Inhibitor for Inflammation and CNS Diseases
Insilico Medicine, a clinical-stage generative AI-driven drug discovery company, has announced the nomination of its 21st pre-clinical candidate, ISM8969, an orally available inhibitor of NLRP3 (NOD-like receptor family pyrin domain containing 3).
This milestone follows Insilico’s recent achievements, including a groundbreaking AI-enabled oncology milestone with Sanofi, the FDA IND clearance of ISM5939 for solid tumors, and the rapid progress of ISM5411, a gut-restricted PHD inhibitor for inflammatory bowel disease.
The candidate is powered by Insilico’s proprietary Pharma.AI platform and designed to cross the blood-brain barrier (BBB), opening potential treatments for a range of inflammation-related and central nervous system (CNS) diseases, including gout flare, asthma, Crohn’s disease, Alzheimer’s disease, and epilepsy.
See also: Insilico Medicine’s Generative AI Patent Provides Advantage in AI Drug Discovery Race
NLRP3 plays a critical role in the immune system by forming the inflammasome, a multimolecular complex that activates innate immune responses. Overactivation of NLRP3 leads to excessive pro-inflammatory cytokine production, causing damage in various diseases. Despite its importance, no FDA-approved therapies currently target NLRP3.
Key Features of ISM8969
ISM8969 demonstrates the following characteristics:
- BBB Penetrability: Unlike many other candidates, ISM8969 can cross the blood-brain barrier, offering therapeutic potential for neuroinflammatory conditions.
- Promising Preclinical Profile: Balanced druggability with in vitro and in vivo efficacy in inflammation and chronic disease models.
- AI-Powered Design: Leveraging Pharma.AI to optimize both biological and chemical properties.
Advancing AI-Driven Drug Discovery
Since 2016, Insilico Medicine has been a pioneer in applying generative AI to molecular design. The company’s Pharma.AI platform has enabled the nomination of 21 pre-clinical candidates and secured IND clearances for 10 molecules.
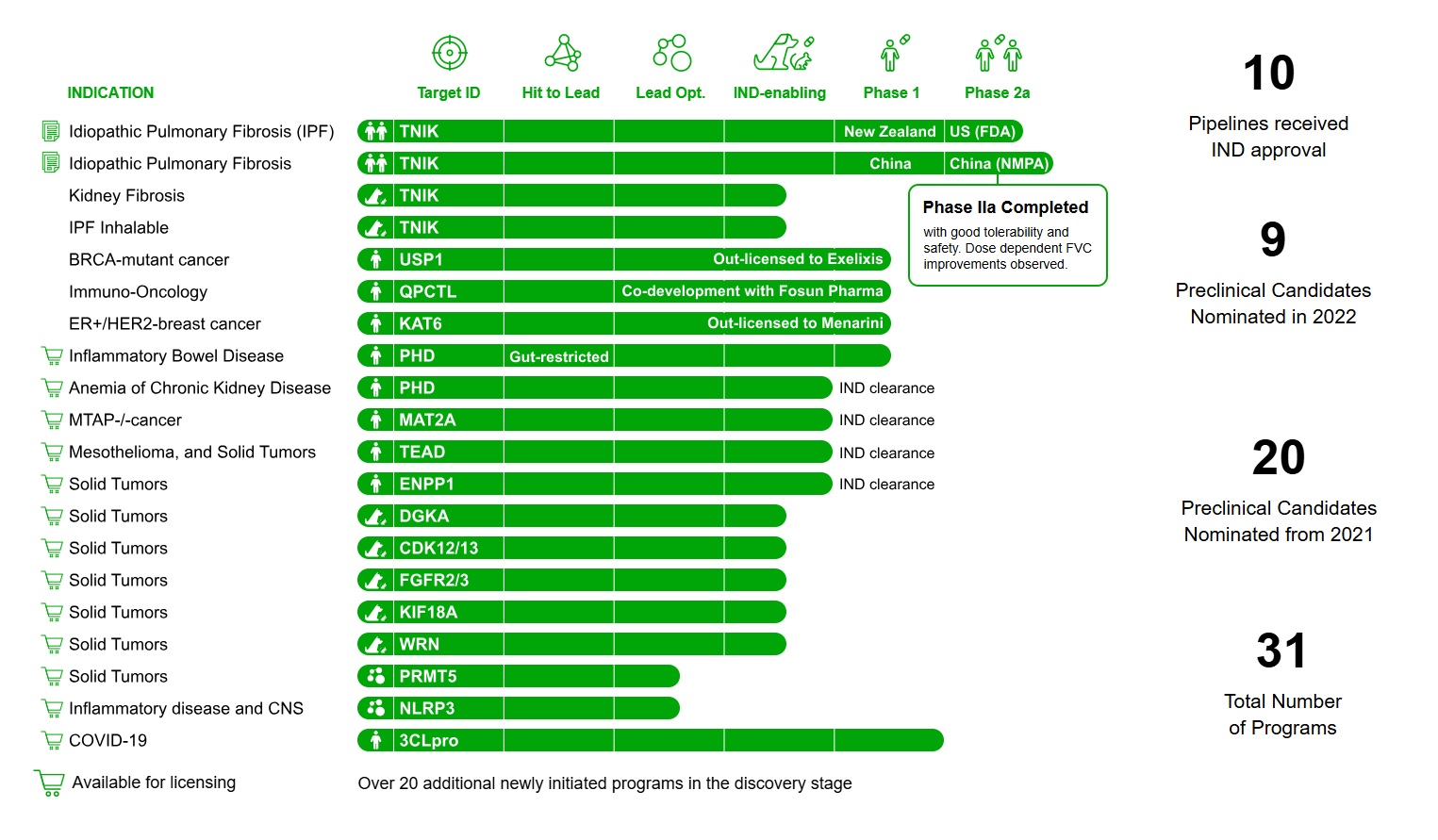
Image credit: Insilico Medicine
Topics: Novel Therapeutics