Citryll Secures €85M to Advance Monoclonal Antibody Targeting NETs in Inflammatory Diseases
Citryll, a biotechnology company focused on developing monoclonal antibody therapies to address immune-mediated inflammatory diseases, has completed an €85 million Series B financing round.
The round was co-led by Johnson & Johnson Innovation – JJDC, Inc., Forbion, and Novartis Venture Fund, with participation from Pureos Bioventures and existing investors including BioGeneration Ventures, Seventure Partners, BOM, Curie Capital, and the company’s founders. The funding will support the clinical development of its lead candidate, CIT-013, and expand its pipeline.
Citryll focuses on targeting Neutrophil Extracellular Traps (NETs), web-like structures released by neutrophils during a process called NETosis, which contribute to chronic inflammation and tissue damage when overproduced. While NETs are part of the immune system’s defense against pathogens, excessive formation can lead to tissue damage and chronic inflammation associated with diseases like rheumatoid arthritis and hidradenitis suppurativa.
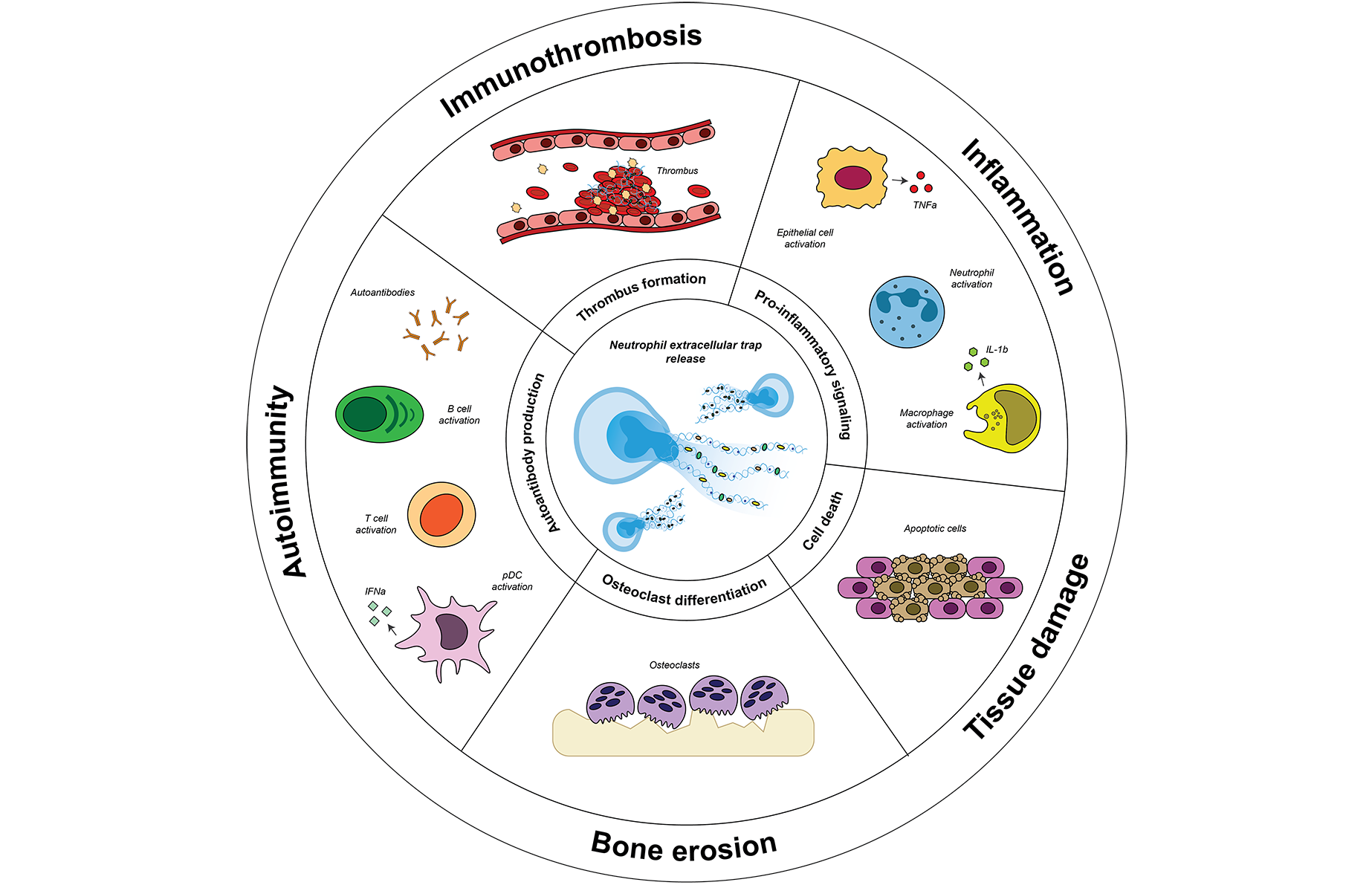
The pathogenic role of NETs, Citryll
The company recently completed Phase 1 trials in rheumatoid arthritis and plans Phase 2a trials for rheumatoid arthritis and hidradenitis suppurativa in 2025.
About CIT-013 and NET-Targeting
CIT-013, a monoclonal antibody, works in two ways to address excessive NETs in inflammatory diseases. First, it binds to specific components of NETs, called citrullinated histones, to stop the formation of new NETs during the final stage of their release. Second, it helps clear existing NETs by forming immune complexes that signal macrophages (a type of immune cell) to engulf and remove them from tissues. This dual action reduces inflammation while preserving the other important functions of neutrophils, the immune cells that produce NETs.
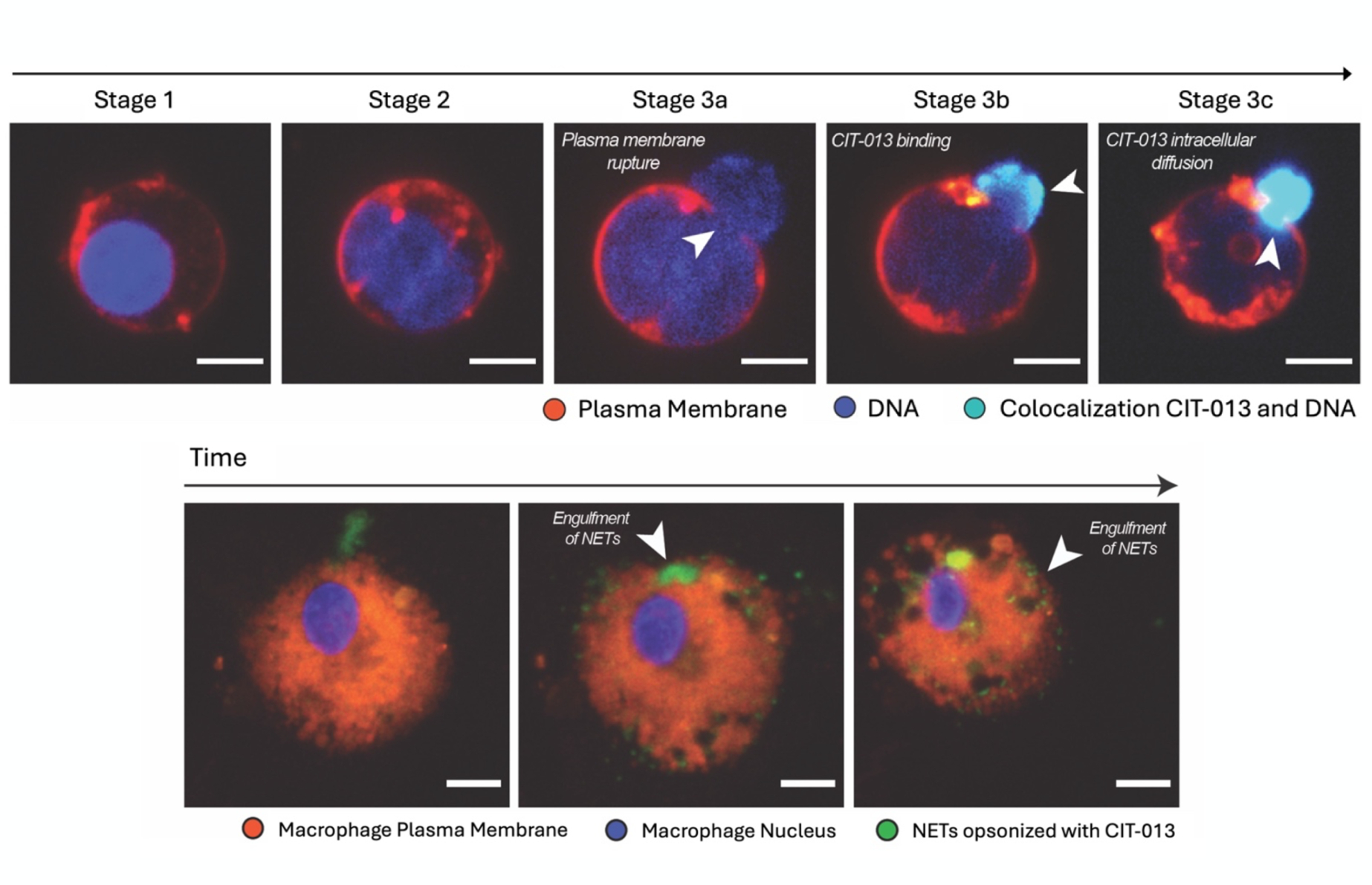
Top: CIT-013 binding to NETs after the NETosis pathway of Neutrophil is activated; Bottom: CIT-013 binding to NET fragments induces phagocytosis by a macrophage; Credit: Citryll
Preclinical studies have shown CIT-013’s ability to suppress NET formation and enhance clearance, offering a novel therapeutic approach to conditions where excessive NETs contribute to disease pathology.
Peer reviewed scientific publications from Citryll can be found in their Publications section.
Clinical Development of CIT-013
Citryll’s clinical program for CIT-013 includes completed and planned trials:
- Phase 1 Studies:
- Single ascending dose and LPS challenge studies in healthy volunteers demonstrated target engagement, safety, and good pharmacokinetics (2022).
- Subcutaneous single dosing and repeat dosing in RA patients confirmed tolerability and bioavailability (2023–2024).
- Phase 2a Trials:
- Planned for rheumatoid arthritis in Q2 2025.
- Planned for hidradenitis suppurativa in Q3 2025.
Beyond these initial indications, the company sees potential applications in other immune-mediated inflammatory diseases where NETs play a pathogenic role.
Geert-Jan Mulder, Managing Partner at Forbion:
“Citryll’s approach offers a novel way to address immune-mediated disorders where current treatments often fall short. We are proud to support this differentiated program through clinical development.”
Eduardo Bravo, CEO of Citryll:
“The support from our global investors enables us to advance our clinical program and explore the broad potential of NET-targeting therapies in inflammatory diseases.”
Topics:
Startups & Deals Novel Therapeutics

