The AI-driven Development Journey of ISM5411, a Potential Breakthrough Treatment for Inflammatory Bowel Disease
Inflammatory Bowel Disease (IBD), a chronic and debilitating autoimmune disorder that includes ulcerative colitis and Crohn’s disease, impacts millions of lives globally. Characterized by chronic gut inflammation, intestinal barrier dysfunction, and increased risk of colitis-associated cancer, IBD remains an unmet clinical challenge. Current treatments, largely focused on anti-inflammatory drugs, offer symptomatic relief but fail to address epithelial barrier damage—a core issue in IBD progression. As a result, patients face the threat of disease recurrence, prolonged dependence on medications, and increased risk of colorectal cancer.
A shift in the therapeutic paradigm is now emerging. The focus is turning toward drugs that actively repair the intestinal barrier while simultaneously reducing inflammation. One promising strategy lies in modulating the Hypoxia-Inducible Factor (HIF) pathway by inhibiting prolyl hydroxylase domain (PHD) enzymes. While this approach has shown potential, prior attempts to develop systemic PHD inhibitors for IBD have failed due to significant off-target effects, notably cardiovascular and tumorigenic risks.
But the story of ISM-5411 (also known in earlier studies as ISM012-042) is different. It revolves around innovation powered by artificial intelligence (AI), a generative chemistry platform, and a risky but seemingly rewarding approach to drug development, involving a high stakes bet on novel targets and design workflows. Developed using Insilico Medicine's AI-driven Pharma.AI platform and its Chemistry42 engine, ISM-5411 might be one of the vivid examples redefining how small molecules are discovered and developed for complex diseases like IBD.
The Role of PHD Inhibitors in IBD Treatment
Prolyl hydroxylases (PHDs) regulate the stability of hypoxia-inducible factors (HIFs), a family of transcription factors that control genes essential for epithelial integrity, tight junction formation, and immune regulation. The HIF-PHD axis plays a critical role in intestinal homeostasis, with PHD inhibition stabilizing HIF proteins to promote barrier repair and immune balance.
PHDs, however, present a “double-edged sword.” While their inhibition supports epithelial barrier repair, systemic inhibition activates HIFs in tissues beyond the gut, leading to increased erythropoiesis and vascular endothelial growth factor (VEGF) production, raising cardiovascular risks. This challenge was exemplified by the clinical failure of GB004, a systemic Fe(II)-chelator-based PHD inhibitor, in Phase 2 trials for IBD. Systemic PHD inhibition became synonymous with safety risks, leaving the industry in need of a better approach.
The challenge was clear: develop a gut-restricted PHD inhibitor. A drug that stays in the gut, acts locally, and avoids the systemic side effects of its predecessors. This is where Insilico Medicine entered the scene.
The Birth of ISM-5411 – From Concept to Candidate
In 2021, Insilico Medicine launched a bold mission: design a novel PHD inhibitor that overcomes the limitations of systemic inhibitors.
The development path was unconventional but proved to be efficient. Insilico began by using its PandaOmics platform to identify PHD1 and PHD2 as viable drug targets for IBD. This choice was guided by data-driven insights into the genes’ role in barrier repair, immune suppression, and inflammation control.
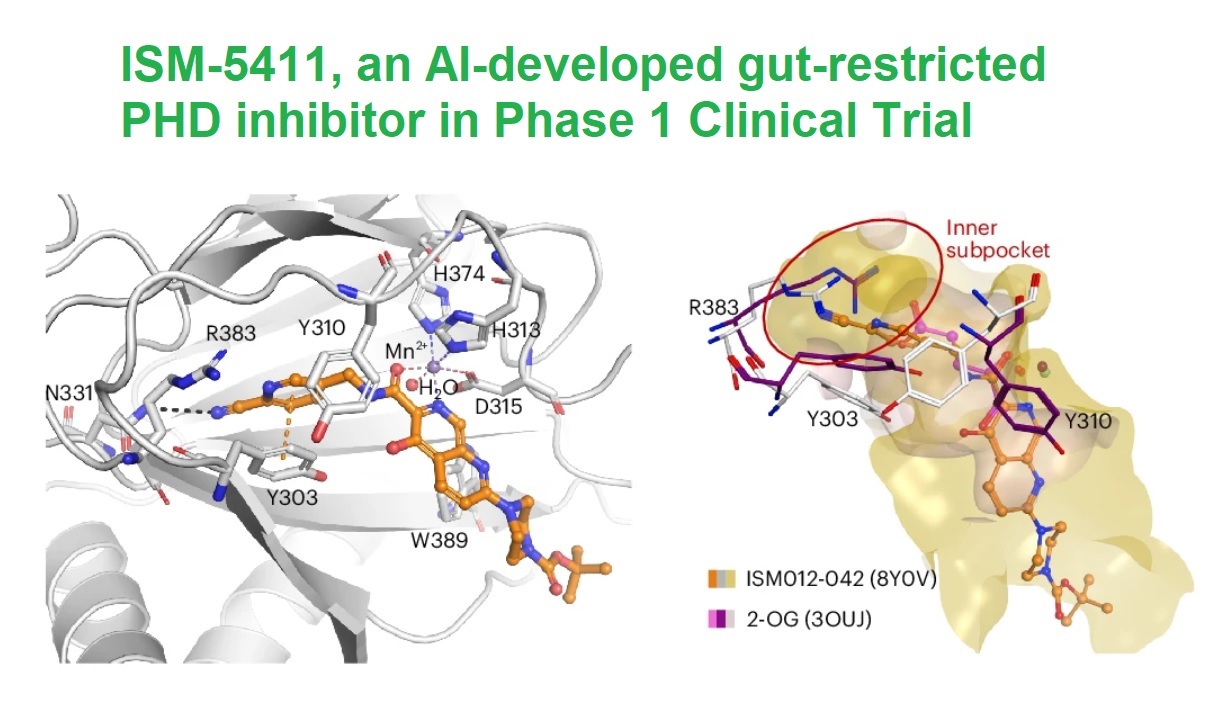
The team then harnessed Chemistry42 to initiate structure-based drug design effort. Using known PHD2 complex structures (like those with Takeda-17, JPHM-2-16, and Molidustat) as templates, Insilico's scientists defined six critical pharmacophore points and seeded a privileged fragment (a benzonitrile group) as the chemical starting point.
This starting point was subjected to fragment-based growth using Chemistry42’s structure-based generative model. The process involved the iterative generation of thousands of potential PHD inhibitors, with AI-driven models filtering candidates for novelty, drug-likeness, and synthetic accessibility.
Hit generation and lead optimization followed, supported by SAR (structure-activity relationship) analysis and potency refinement using Alchemistry, which calculates binding free-energy estimates for PHD2-ligand complexes. Next, ADMET profiling predicted key drug properties like solubility, permeability, and systemic exposure risk. The result of this process was a single lead candidate: ISM-5411.
Within 12 months, Insilico had to synthesize and screen only 115 molecules to nominate a preclinical candidate, which seems remarkable in terms of effort/result ratio, which might indicate efficiency of its computational platform.
A Speedy Path To Clinical Trials
ISM012-042 stands out for its unique mechanism and pharmacological profile. The compound selectively inhibits PHD1 and PHD2 with IC50 values of 1.9 nM and 2.5 nM, respectively, while showing minimal activity on PHD3. This specificity addresses safety concerns, as PHD3 inhibition is linked to increased tumorigenic risk.
The most significant aspect of ISM012-042 is its gut-restricted pharmacokinetics. It remains in the colon, minimizing systemic exposure. Animal models confirmed a 67-fold higher concentration in the colon than in plasma, ensuring local activity where it is needed most. Pharmacokinetic analysis in rats revealed that nearly 85% of the compound was excreted in feces, reflecting low systemic absorption.
The compound's unique binding mechanism further distinguishes it from competitors. Unlike Fe(II) chelators like GB004, ISM012-042 binds PHD2 directly, inducing a conformational change that stabilizes HIF-1α, even in the presence of high iron concentrations. This feature eliminates the "iron dependence" of prior inhibitors.
To demonstrate efficacy, Insilico tested ISM012-042 in two experimental colitis models:
- TNBS-induced colitis model (driven by chemical irritation)
- Oxazolone-induced colitis model (mimicking human Th2-driven colitis)
Results were strong. ISM012-042 outperformed mesalamine, the current standard of care, in reducing disease activity index (DAI) scores and improving epithelial integrity. Colonic tissue samples revealed increased tight junction protein (ZO-1) retention and elevated expression of barrier-protective genes like TJP1, CD73, and TFF3. Cytokine profiling showed reductions in IL-6, IL-17, and TNF-α, while immune cell infiltration of neutrophils and pro-inflammatory T cells was significantly reduced. These effects were dose-dependent, supporting its potential for both preventative and therapeutic applications in IBD.
Armed with compelling preclinical data, Insilico Medicine submitted an Investigational New Drug (IND) application for ISM012-042. Approval was granted, leading to the initiation of a Phase 1 clinical trial (NCT06012578) in late 2023. This trial aims to assess the drug’s safety, pharmacokinetics, and pharmacodynamics in healthy human volunteers.
With its gut-restricted exposure, ISM012-042 is expected to avoid the cardiovascular and tumorigenic risks that derailed GB004. Early indicators suggest that the compound may finally achieve what other PHD inhibitors could not—a safe, effective, and gut-specific treatment for IBD.
Topics: Novel Therapeutics