Cradle Achieves 8x Improved EGFR Binding Over Merck’s Cetuximab in Protein Design Challenge
Cradle’s platform has achieved first place in Adaptyv Bio's protein design competition by producing a novel EGFR-binding protein with a binding affinity of 1.21 nM, outperforming Merck’s Cetuximab (9.94 nM) by 8-fold. This competition featured 130 teams and 1,131 designs, with only two outperforming Cetuximab.
The Competition
Adaptyv Bio’s protein design competition tasked 130 teams with designing innovative binders for EGFR (Epidermal Growth Factor Receptor), a key target in cancer therapies for conditions such as non-small cell lung cancer and colorectal cancer. The competition aimed to push the boundaries of protein engineering by requiring designs that differed significantly from known antibodies, including Cetuximab.
Participants submitted 1,131 designs, of which 400 underwent lab testing using Surface Plasmon Resonance (SPR). Most competitors utilized tools like RFdiffusion or BindCraft, while Cradle leaned on its proprietary platform for the design process. Only two designs surpassed Cetuximab in binding affinity, with Cradle achieving the best result by a significant margin.
See also: Cracking the Code of Life: An Interview with Stef van Grieken, CEO of Cradle
Strategy: Precision Optimization
Rather than employing a de novo design approach, Cradle optimized an existing protein—the scFv-format Cetuximab. A strategic decision was made to shield the antibody's CDR (Complementarity-Determining Regions), critical for binding, from modifications. This allowed the platform to focus on optimizing the surrounding framework regions, which often influence stability and binding strength.
Credit: Cradle
Cradle’s AI-driven platform automated the entire process:
- Framework regions were selected for optimization based on structural insights.
- Mutations were proposed and scored using Cradle’s models.
- Designs were finalized and submitted overnight without manual intervention.
This approach proved crucial, as traditional methods often focus heavily on CDR engineering, whereas Cradle’s platform identified additional pathways to enhance binding affinity.
The Results
Cradle’s final design demonstrated:
- A binding affinity (KD) of 1.21 nM, compared to 9.94 nM for Cetuximab and 5.18 nM for the closest competitor.
- Robust in-vitro performance confirmed via SPR testing.
Cradle’s entry initially ranked low (300-600 range) in in-silico scoring due to competition mechanics favoring shorter sequences. Despite this, the experimentally validated results underscored the importance of real-world testing over computational rankings.
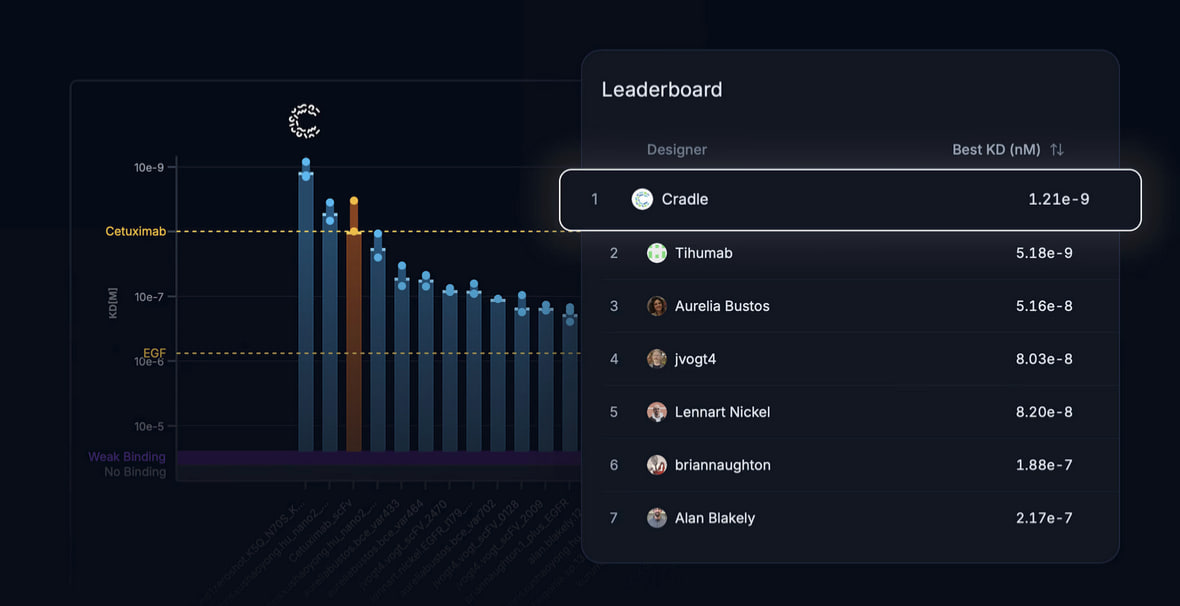
Credit: Cradle
Improved binding affinity is an important early step in the process of drug discovery. However, turning a high-performing binder into a viable therapeutic candidate requires addressing additional factors such as stability, manufacturability, and immunogenicity. These aspects ensure the molecule can function effectively in clinical settings, can be produced at scale, and is safe for patient use.
See also: Navigating Next Wave of Protein Design with Elise de Reus from Cradle
There's inherent complexity to the therapeutic development, as multiple properties must be optimized simultaneously. The competition result is a promising milestone, but further iterative design and testing would be necessary to advance toward clinical applications.
Topics: Biotech