Vittoria Biotherapeutics Highlights Novel CAR-T Cell Therapy Approach at ASH 2024
Vittoria Biotherapeutics, a Philadelphia-based clinical-stage immunotherapy company, showcased preclinical findings at the 66th Annual American Society of Hematology (ASH) Meeting.
Key findings were presented in two studies:
The oral presentation, titled "CD5 Elimination Enhances CART Cell Proliferation Through JAK-STAT Activation" demonstrated:
“While previous preclinical studies have found that the elimination of CD5 can result in increased CAR-T cell activity, the molecular mechanisms accounting for the change have remained unclear. Bulk RNA sequencing analysis found that the elimination of CD5 led to enhanced signaling in JAK-STAT, MAPK, and mTOR pathways, primarily through increases in the expression of cytokines and growth factors. These results establish a clear mechanism accounting for the increased proliferative capacity and antitumor activity associated with eliminating CD5 and support Vittoria’s broader approach.”
The poster presentation, titled "Evaluating Efficacy and Safety of Rapid-Manufactured CD5KO CART5 Cells in Preclinical Models of T Cell Malignancies" revealed:
“This study evaluated the impact of different manufacturing processes on the activity of CD5 knockout CAR-T cells. Notably, large-scale rapid manufacturing, utilizing Vittoria’s proprietary process, demonstrated significantly enhanced anti-tumor responses, improved survival rates, and superior T cell expansion in rodent models of T-cell lymphoma compared to conventional small-scale manufacturing methods. Additionally, the large-scale rapid product exhibited a favorable safety profile across multiple metrics in preclinical rodent models.”
These findings validate the company’s proprietary Senza5 platform, which is designed to enhance the efficacy and durability of engineered CAR-T cell therapies by targeting the immunomodulatory protein CD5.
The Senza5 Platform
Vittoria’s Senza5 platform is central to its efforts to create next-generation autologous CAR-T cell therapies. The platform disables CD5 signaling to overcome immunosuppression and amplify antitumor activity. It employs a five-day manufacturing process that reduces production timelines while enhancing T-cell stemness, promoting in vivo expansion, and extending durability.
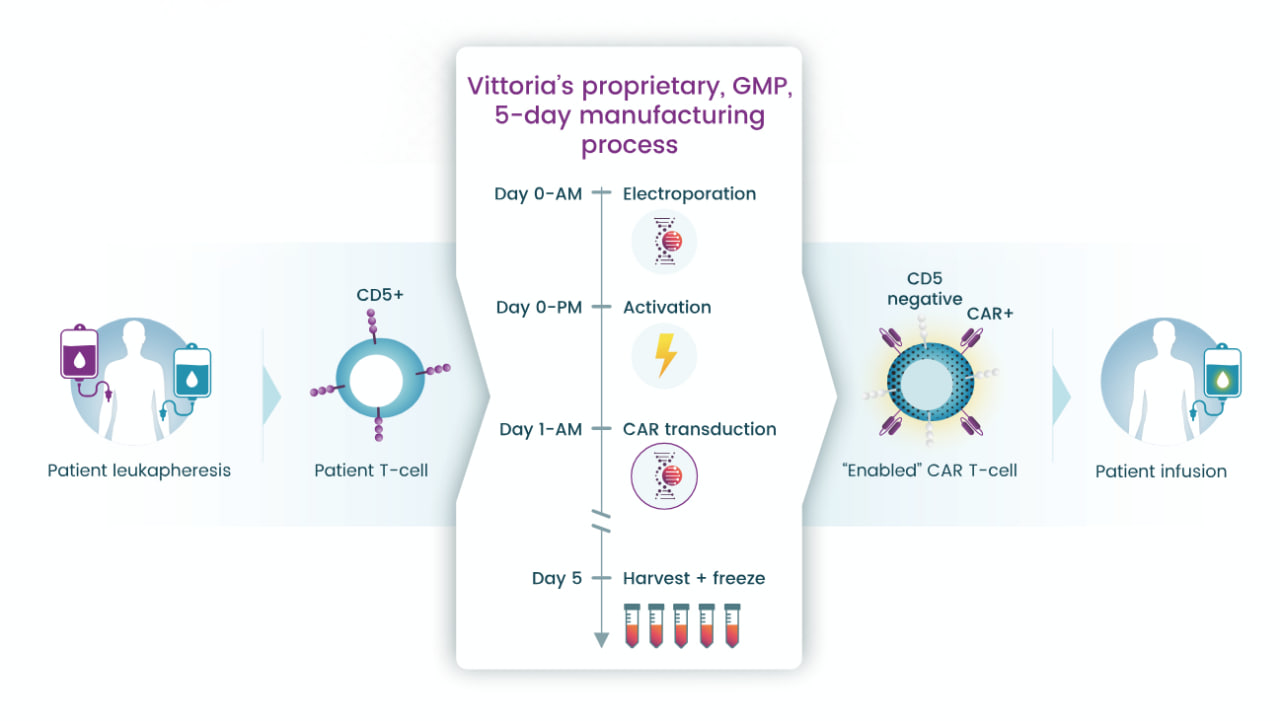
Vittoria's Senza5 technology manufacturing process; Source: Vittoria Biotherapeutics
Reportedly, laboratory studies have shown that Senza5-engineered CAR-T cells significantly outperform conventional CAR-T cells in preclinical models of both liquid and solid tumors. By optimizing T-cell activation, cytotoxicity, and resistance to exhaustion, Senza5 provides a robust foundation for advancing therapies targeting difficult-to-treat cancers and autoimmune diseases.
Pipeline Advancements
Vittoria Biotherapeutics is advancing a pipeline anchored by VIPER-101, a gene-edited CAR-T therapy for T-cell lymphoma. VIPER-101 targets CD5, a protein present on cancer cells in over 85% of T-cell lymphoma cases, and has shown superior efficacy in preclinical models. This therapy is currently being evaluated in a Phase 1 clinical trial.
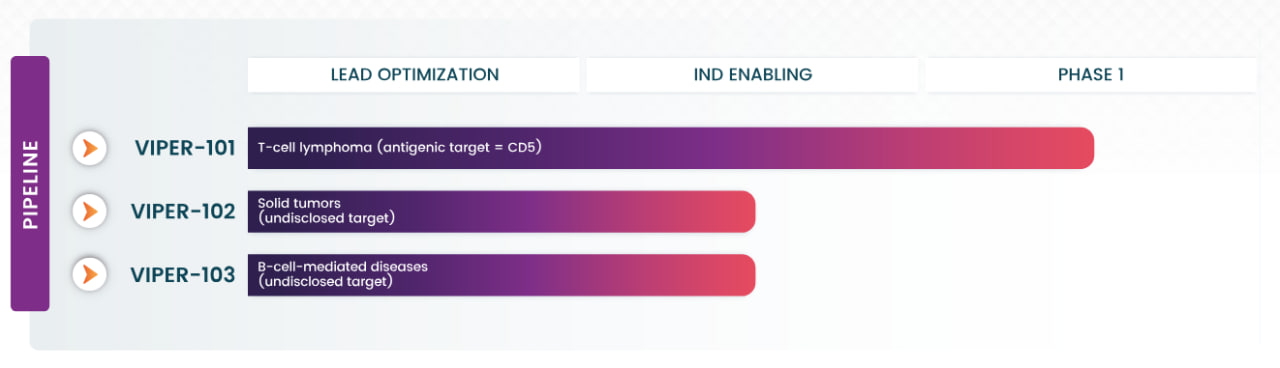
Vittoria's current pipeline; Source: Vittoria Biotherapeutics
The company is also developing VIPER-102 for solid tumors and VIPER-103 for B-cell-mediated autoimmune diseases. Both programs utilize CAR-T cells engineered to target novel antigens with high disease specificity, aiming to overcome challenges like off-tumor toxicities and improve safety profiles.
Cover image: Maksim Tkachenko
Topics: Novel Therapeutics