Insilico Medicine Celebrates Its 10th AI-Driven Drug Candidate to Get FDA IND Clearance
Insilico Medicine has announced FDA IND clearance for ISM5939, marking the 10th AI-designed drug candidate from the company to enter clinical trials. The molecule, a novel oral small molecule inhibitor targeting ENPP1, is designed for the treatment of solid tumors.
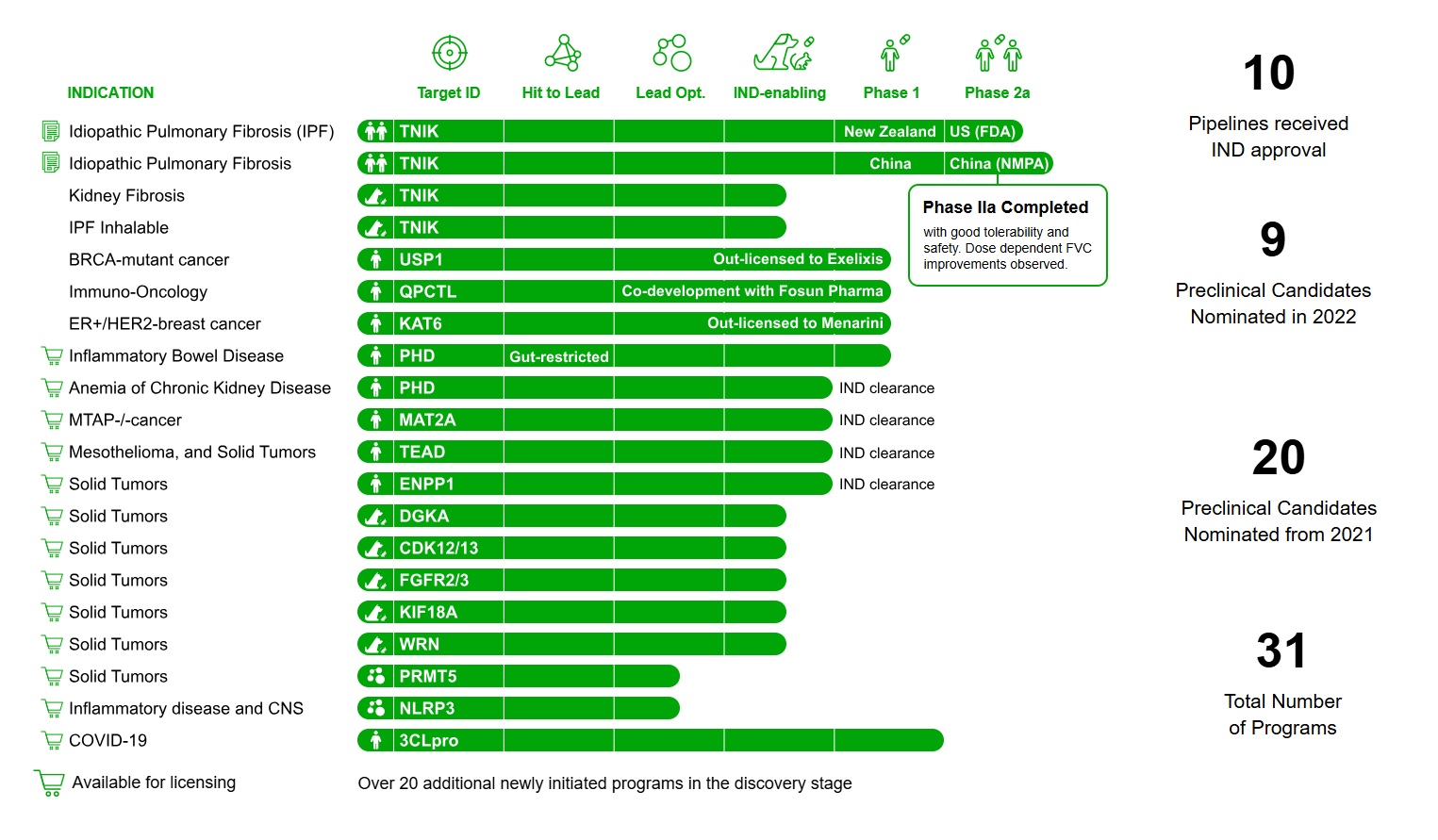
Image credit: Insilico Medicine
ISM5939 works by regulating extracellular cGAMP levels to activate the cGAS-STING pathway, enhancing the anti-tumor response of the host immune system. Elevated ENPP1 expression has been linked to metastasis and poor prognosis in several tumor types. Preclinical studies demonstrated robust anti-tumor efficacy, a favorable safety profile, and promising pharmacokinetic properties.
The drug candidate was designed and optimized using Chemistry42, part of Insilico’s proprietary AI-driven Pharma.AI platform. The lead compound was developed in just three months, reflecting the efficiency of the AI platform.
Since 2021, Insilico Medicine has nominated 20 preclinical candidates and achieved 10 IND clearances across its pipeline of over 30 drug discovery programs. Earlier in 2024, Insilico published a Nature Biotechnology paper detailing the journey of its flagship molecule, ISM001_055, which advanced from AI algorithms to Phase II clinical trials.
ISM001_055 has recently demonstrated positive preliminary results in a Phase IIa trial (NCT05938920), showing favorable safety and tolerability as well as dose-dependent efficacy in improving forced vital capacity (FVC).