Axsome Therapeutics Announces Positive Phase 3 Results for Reboxetine in Narcolepsy
Axsome Therapeutics announced that its investigational treatment, AXS-12 (reboxetine), met the primary endpoint in the ENCORE Phase 3 trial. The trial demonstrated that AXS-12 significantly reduced the frequency of cataplexy attacks compared to placebo, alongside improvements in excessive daytime sleepiness (EDS), cognition, and overall narcolepsy symptoms.
Founded in January 2012, Axsome Therapeutics is a U.S.-based biopharmaceutical company listed on NASDAQ under the ticker AXSM. The company focuses on developing novel therapies for central nervous system (CNS) conditions with limited treatment options.
With a pipeline that includes late-stage candidates addressing narcolepsy (AXS-12), Alzheimer’s disease agitation (AXS-05), migraine (AXS-07), and fibromyalgia (AXS-14), the company focuses on therapies with novel mechanisms of action to expand options for patients and physicians.
Axsome currently markets Sunosi (solriamfetol) for excessive daytime sleepiness and Auvelity, a combination of dextromethorphan and bupropion, for major depressive disorder.
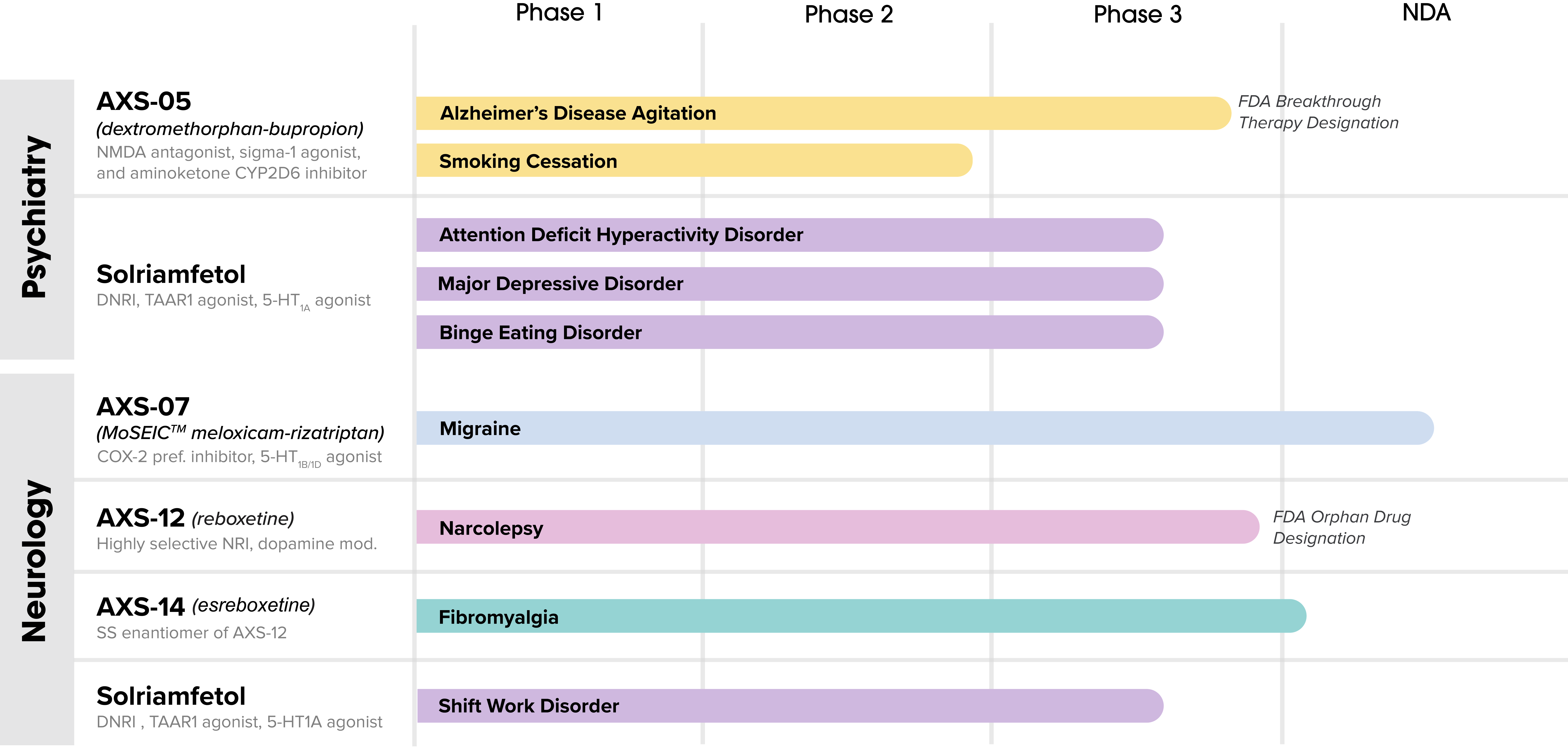
Axosome's current pipeline
Trial Results
In the ENCORE trial, which included a 24-week open-label treatment phase followed by a randomized withdrawal period, AXS-12 demonstrated robust efficacy. Patients continuing treatment with AXS-12 during the withdrawal phase reported an increase of only 1.32 weekly cataplexy attacks compared to 10.29 attacks in the placebo group (p=0.017). Long-term data showed sustained benefits, with a 77% reduction in weekly cataplexy attacks after six months and 82% of patients achieving at least a 50% improvement. Cognitive and EDS improvements were also maintained, with over 78% of patients reporting sustained benefits.
AXS-12 was reported to be well-tolerated, with no new safety signals identified. Adverse events were consistent with prior studies, and discontinuations due to side effects were minimal. These results pave the way for Axsome to submit a New Drug Application (NDA) to the FDA.
Topics: Clinical Trials