Novo Holdings Supports $135 Million Funding to Advance Adcendo’s Antibody-Drug Conjugate Development
Novo Holdings has joined a $135 million Series B financing round for Adcendo, a clinical-stage biotechnology company headquartered in Copenhagen, Denmark.
Adcendo is focused on developing first-in-class antibody-drug conjugates (ADCs) to address cancers with high unmet medical needs. ADCs combine targeted antibody delivery with potent drug payloads, offering a precision approach to treating difficult-to-manage cancers while minimizing damage to healthy tissue.
Adcendo’s strategy is built on a deep understanding of tumor biology, enabling the identification of novel targets and the development of optimized linker-payload technologies. The company’s lead programs, including ADCE-T02 (targeting Tissue Factor) and ADCE-D01 (targeting uPARAP), are part of a broader pipeline designed to treat solid tumors with limited therapeutic options.
See also: The Rising Popularity of Antibody-Drug Conjugates, with Challenges
A Brief on Antibody-Drug Conjugates
Antibody-drug conjugates are a novel class of cancer therapies that combine the specificity of antibodies with the potent cell-killing capabilities of cytotoxic drugs. Designed to deliver precise, targeted treatment to malignant cells, ADCs are engineered with three key components:
- Antibody: A monoclonal antibody specifically engineered to recognize and bind to antigens overexpressed on the surface of cancer cells. This targeting capability allows the ADC to differentiate cancer cells from normal tissue.
- Payload: A highly cytotoxic drug designed to destroy cancer cells. Once inside the cell, the payload disrupts critical cellular processes, leading to cell death. Payloads are selected for their potency, as only a small amount is needed to effectively kill the cancer cells.
- Linker: A chemical structure that covalently attaches the payload to the antibody. The linker ensures stability while the ADC is circulating in the bloodstream and enables the payload’s release once the ADC is internalized by the target cancer cell.
Upon binding to the target antigen, the ADC is absorbed by the cancer cell, where the linker releases the payload. This release allows the payload to act directly within the cancer cell, minimizing off-target effects and reducing damage to healthy tissues.
ADCs are highly customizable, with a range of available antibodies, payloads, and linkers. This flexibility allows ADCs to be fine-tuned to target specific cancer types and molecular pathways. Over 10 ADCs are currently approved for clinical use, and hundreds more are in development, addressing both solid tumors and hematologic malignancies.
Adcendo’s Leading ADC Candidates
ADCE-D01: uPARAP — A Potential Pan-Sarcoma ADC Target
Adcendo’s ADCE-D01 program targets uPARAP, a cell membrane receptor that presents a promising avenue for treating sarcomas and certain epithelial tumors. uPARAP exhibits unique biological characteristics that make it an ideal candidate for ADC development:
- Target Biology: uPARAP is a constitutively cycling receptor that internalizes ligands and cargo within minutes of binding. It plays a role in tissue homeostasis, including wound healing and immune cell trafficking, by binding and internalizing collagen fragments.
- Expression Profile: The receptor is strongly overexpressed in mesenchymal tumors, such as soft-tissue sarcomas, bone sarcomas, and gastrointestinal stromal tumors (GISTs). It is also found in cancer-associated fibroblasts (CAFs) within the stroma of epithelial tumors, potentially offering therapeutic benefits in a range of cancers.
- Clinical Potential: ADCE-D01 represents the first potential pan-sarcoma ADC targeting uPARAP, addressing a significant unmet need in mesenchymal and stromal-driven tumors.
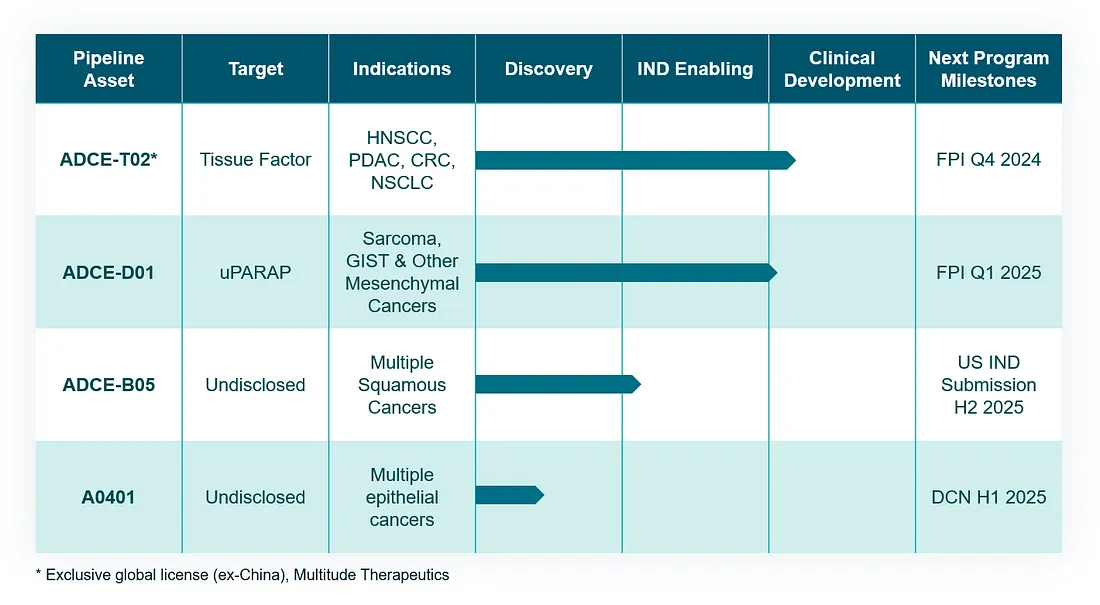
Adcendo’s current pipeline; Credit: Adcendo
ADCE-T02: Tissue Factor — A Highly Attractive ADC Target
The ADCE-T02 program focuses on Tissue Factor (TF), another compelling target for ADC development. TF plays critical roles in tumor progression and is associated with aggressive cancer phenotypes:
- Target Biology: TF is primarily restricted to perivascular tissues under normal physiological conditions and serves as the primary initiator of coagulation. In cancer, however, TF facilitates tumor growth, angiogenesis, invasion, and metastasis.
- Mechanisms Driving Overexpression: TF overexpression in tumors is linked to hypoxia, loss of tumor suppressor genes such as TP53 and PTEN, and heightened MAPK signaling activity.
- Therapeutic Opportunity: Given its tumor-specific overexpression and contribution to cancer progression, TF presents a highly selective and effective target for ADC therapies. ADCE-T02 aims to exploit these features to deliver potent, tumor-localized treatment.
Both ADCE-D01 and ADCE-T02 exemplify Adcendo’s precision approach to developing targeted therapies, leveraging novel biology to address cancers with high unmet needs. These programs highlight the versatility and potential of antibody-drug conjugates to transform treatment paradigms across multiple cancer types.
Cariad Chester, Managing Partner, TCGX, said:
“ADCs have transformed the clinical landscape and standard of care in the treatment of solid tumours. Continued progress for hard-to-treat cancers will require innovative approaches, and I’m confident Adcendo will be a leader in the next era of ADC drug development. With this financing, Adcendo can rapidly advance a pipeline of exciting, differentiated ADC candidates. These programs have the potential to significantly change the treatment paradigm in multiple cancers and serve patients in need of better therapies.”
Novo Holdings, an early supporter of Adcendo, participated in the financing alongside other prominent investors, including TCGX, TPG Life Sciences Innovations, Orbimed Advisors, and RA Capital Management. The funding will enable Adcendo to accelerate the clinical development of its ADC pipeline and strengthen its collaborations.
Topics: Startups & Deals