AI-Driven Drug Shows Promising Phase IIa Results in Treating Fatal Lung Disease
Insilico Medicine, a biotechnology company that developed foundational artificial intelligence (AI) in drug discovery platform, recently reported promising results from a Phase IIa clinical trial of its experimental drug ISM001-055, developed specifically to treat idiopathic pulmonary fibrosis (IPF). The trial results showed that the drug, designed entirely using generative AI, could improve lung function in patients over just 12 weeks, offering a potential new therapeutic option for those battling this life-threatening disease.
The 12-week study demonstrated that ISM001-055 was not only safe and well-tolerated but also produced meaningful improvements in patients' lung capacity and quality of life, especially at higher doses. These early findings hint that this new drug could not only slow the progression of IPF but may potentially reverse some of the damage it causes, a prospect that excites many in the medical community.
Idiopathic Pulmonary Fibrosis: An Urgent Medical Need
IPF is a chronic and progressive lung disease characterized by relentless scarring of the lungs, which reduces their capacity to transfer oxygen to the bloodstream. Affecting nearly 5 million people worldwide, the disease is particularly devastating due to its progressive nature and poor prognosis, with most patients surviving only 3 to 4 years after diagnosis. Current treatments, including antifibrotic drugs, help slow disease progression but don’t stop or reverse it, underscoring the urgent need for better options.
"IPF is a cruel disease. As scarring worsens, patients struggle more with breathing and face a steady decline in quality of life," said Dr. Toby M. Maher, a lead investigator in the ISM001-055 study. "Seeing lung function improve in just 12 weeks is something we haven’t observed often, and it’s a very encouraging sign."
Generative AI and Drug Discovery: Insilico’s Innovative Approach
ISM001-055 stands out not only because of its potential impact on IPF but also because of how it was developed.
Pharma.AI -- the end-to-end drug discovery solution platform based on generative AI.
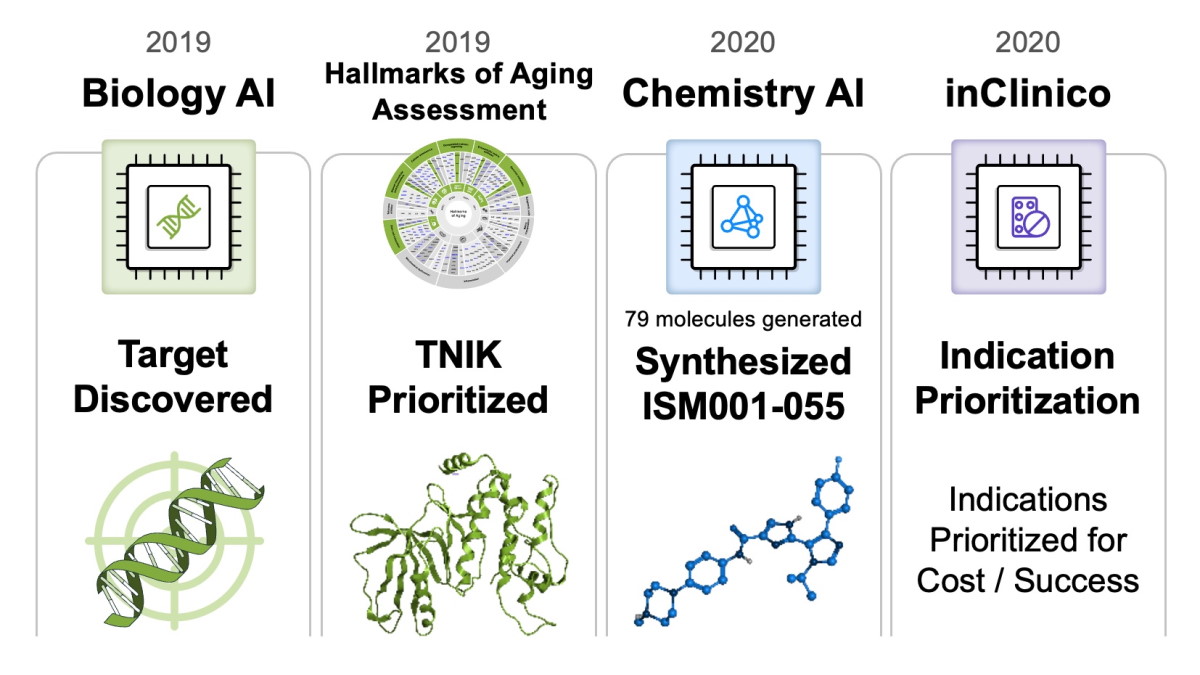
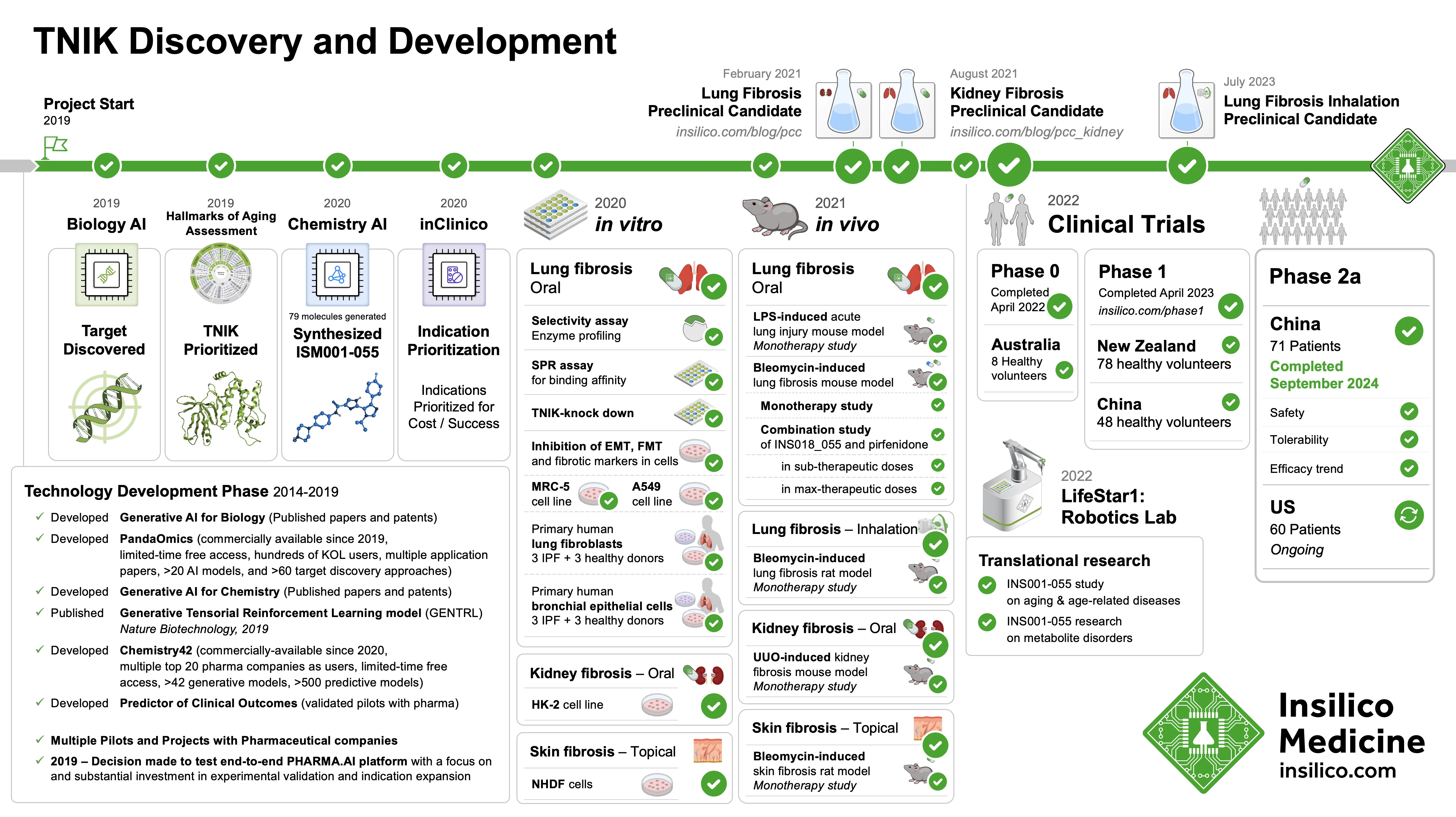
Insilico’s Pharma.AI platform, a generative AI-based system, was responsible for identifying TNIK (Traf2- and NCK-interacting kinase) as a promising target in IPF.
The drug was then designed to inhibit this kinase, which plays a role in the pathological fibrosis characteristic of IPF. By blocking TNIK, Insilico aims to prevent, halt, or potentially reverse the lung scarring that defines the disease.
Dr. Alex Zhavoronkov, CEO of Insilico Medicine, elaborates: "Our goal is to accelerate drug discovery and development using AI, and ISM001-055 is a testament to how powerful these tools can be. AI allows us to design molecules with a higher probability of success and to develop them more efficiently and cost-effectively."
Trial Results: Hope on the Horizon for IPF Patients
The recent trial enrolled 71 patients across 21 sites in China, testing ISM001-055 at different doses against a placebo. Patients were administered either a placebo, 30 mg once daily (QD), 30 mg twice daily (BID), or 60 mg QD for 12 weeks. The findings were compelling:
- Lung Function Improvement: Patients treated with the highest dose (60 mg QD) saw a 98.4 mL increase in forced vital capacity (FVC), a critical measure of lung function, compared to a decline of -62.3 mL in the placebo group. This improvement in FVC at higher doses indicates a dose-dependent response, meaning that the drug’s effectiveness increased with higher doses.
- Quality of Life Gains: The same high-dose group also reported a 2-point improvement in the Leicester Cough Questionnaire (LCQ) score, a measure of cough severity and its impact on quality of life, which is significant for IPF patients who often struggle with chronic, debilitating cough.
- Safety and Tolerability: The drug was generally well-tolerated across all dosing levels, with the most common side effects being mild gastrointestinal issues, such as diarrhea, and some instances of mild liver function abnormalities. Overall, patients tolerated ISM001-055 well, even at higher doses.
These findings support the idea that ISM001-055 could change the treatment paradigm for IPF, offering hope for not just slowing the disease but potentially reversing some of its damage. "The dose-dependent improvement in lung function is incredibly promising," noted Dr. Zuojun Xu, the study's principal investigator, adding, "This could be the start of a much-needed change in how we treat IPF."
Next Steps and Regulatory Pathway
With these positive results, Insilico Medicine is already preparing to initiate discussions with regulatory agencies to plan a pivotal Phase III trial, which would enroll a larger number of patients and further validate ISM001-055’s effectiveness. Additionally, the company plans to present the full Phase IIa data at upcoming medical conferences and submit it for publication in a peer-reviewed journal.
Dr. Carol Satler, a veteran clinical development leader, recently joined Insilico Medicine as Vice President of Clinical Development to oversee the progression of ISM001-055 and other non-oncology programs. "We see ISM001-055’s potential to truly make a difference for IPF patients," Dr. Satler explained. "Future studies will allow us to confirm the drug’s disease-modifying potential, and with luck, provide a new therapeutic option for IPF patients worldwide."
A Future for AI in Drug Discovery
Insilico Medicine’s work on ISM001-055 is part of a broader mission to apply AI to every phase of drug discovery and development. The company’s AI platform, Pharma.AI, has already generated dozens of promising drug candidates, nine of which have received Investigational New Drug (IND) approval. The journey began in 2016, when Insilico first described its concept of using generative AI for molecule design, paving the way for its flagship platforms, PandaOmics for biological insights and Chemistry42 for molecular generation.
ISM001-055 is the company’s latest and most advanced example of the potential for AI in drug discovery, underscoring how these technologies can produce therapies for complex diseases with speed, accuracy, and cost-efficiency not achievable with traditional methods. With early successes like this, Insilico Medicine is positioning itself as a leader in AI-powered biopharma, aiming to bring new hope to patients with complex and challenging diseases like IPF.
As Insilico Medicine prepares to advance ISM001-055 into larger trials, the medical community remains cautiously optimistic, but hopeful, that this AI-developed drug might finally offer relief to millions suffering from IPF worldwide.