BC Platforms Unveils Platform to Unify EU Healthcare Data Governance
BC Platforms has launched its EHDS-Ready Trusted Research Environment (TRE), a platform designed to help EU healthcare institutions comply with European Health Data Space (EHDS) regulations beginning in 2025. EHDS aims to unify EU healthcare data governance, supporting both primary and secondary patient data use, and is projected to save €5.5 billion over a decade by improving data exchange efficiency across Europe.
See also: AI Drug Discovery: Unleashing the Potential of Big Data and Machine Learning
BC Platforms' TRE solution, compatible with various data standards like OMOP CDM, FHIR, and openEHR, allows healthcare data to be securely accessible and interoperable across EU borders. The platform supports real-world data (RWD) analysis, which is critical for research and policy in the healthcare sector. As anticipated EHDS regulations roll out, BC Platforms’ TRE enables federated data analysis, facilitating cross-border research while maintaining strict security standards.
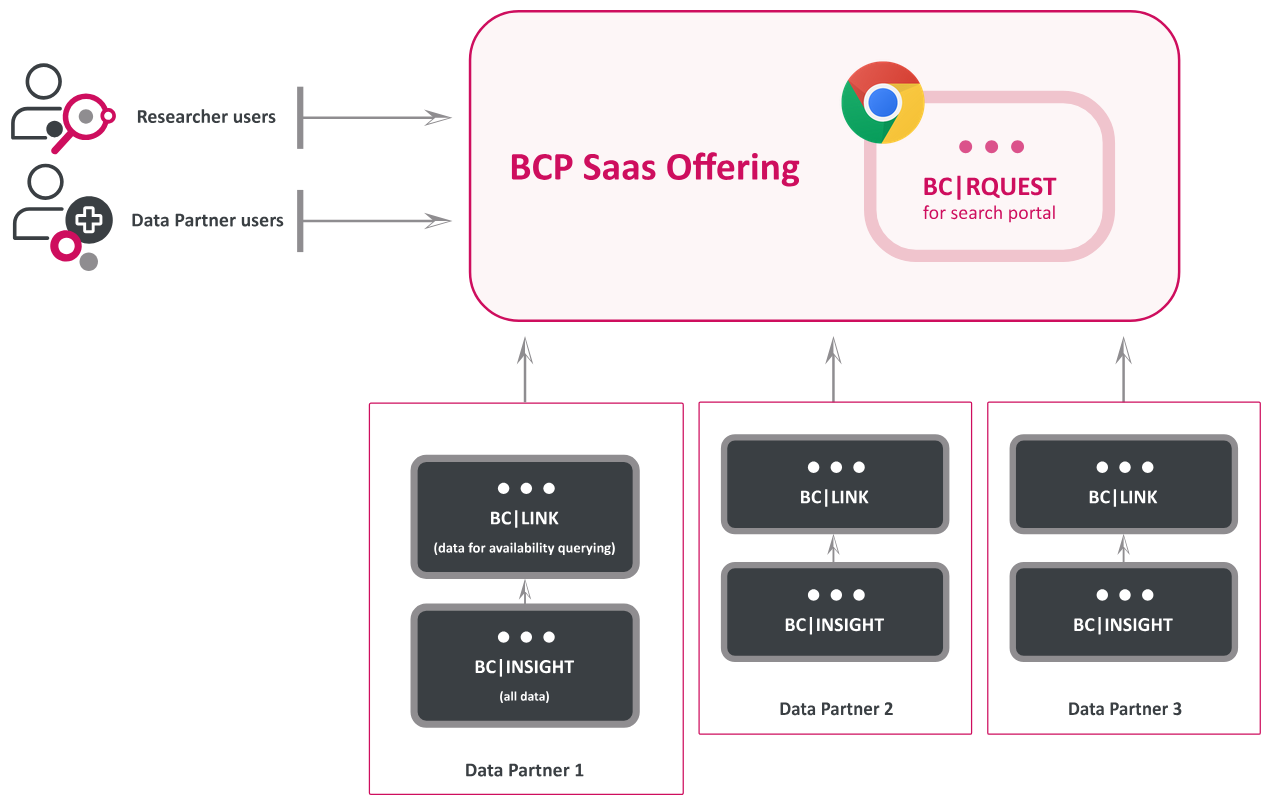
From BC Platforms' presentation: "While data does not leave the original environment, approved users are allowed to access..."
Mikaela Bruhammar, Interim CEO, explained:
"Our platform provides EU healthcare institutions with the digital capabilities required to meet EHDS needs within a limited timeframe, ensuring the EU’s viability as a global healthcare data provider."
Timo Kanninen, Chief Scientific Officer, elaborated on the demand for scalable solutions:
"With EHDS, the need of the healthcare sector across the EU will be almost exponential in scope. Our initial calculations indicate that, in Finland alone, data queries will increase by approximately 25 times—a substantial increase that will require new solutions."
Since 1997, BC Platforms has specialized in secure data environments and invites healthcare institutions across the EU to discuss EHDS-compliant collaborations.
Topics: HealthTech